📣 Updates from the ADA 2022 – Part 2
Jul 12, 2022The previous update of the ADA 2022 discussed how closed-loop systems like the MiniMed 780G, Control IQ, and open-source systems will make life easier for people with diabetes. This time I discuss key lessons learned from studies with 2 other closed-loop systems (CamAPS FX and Beta Bionics), as well as an important update on some next-generation CGMs (continuous glucose monitors):
- Cam APS FX cannot stop the decline of C-peptide in type 1 diabetes
- Fiasp provides little added value with the iLet insulin-only from Beta Bionics
- MARD update: Eversense 365 scores 9.8% and Simplera 10.8%
Curious about the best novelties of the ADA? Then read on quickly!
Get Access To Updated Diabetes Technology Courses
1️⃣ Closed-loop technology cannot stop the decline of C-peptide in type 1 diabetes (Cloud RCT with CamAPS FX)
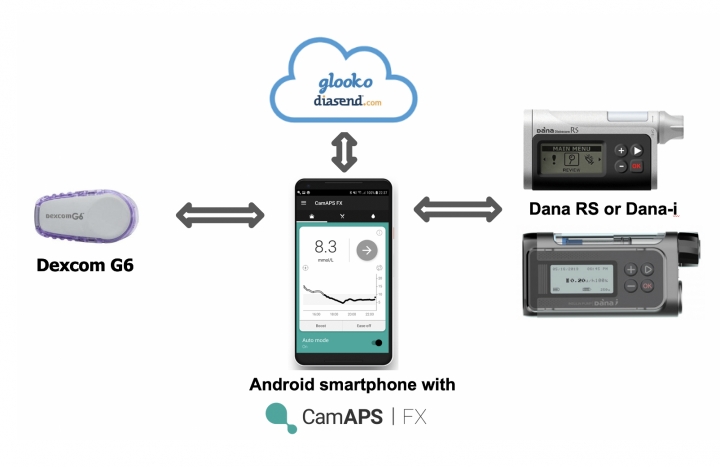
Type 1 diabetes results from a progressive autoimmune breakdown of beta cells in the pancreas, which continues after diagnosis. Residual endogenous insulin production is measured in the blood via C-peptide (a polypeptide produced by beta cells during the production of insulin).

Some people with type 1 diabetes have a measurable C-peptide for an extended period of time after their diagnosis. These people have better glycemic control, a reduced risk of hypoglycemia and a lower risk of microvascular complications. There is a hypothesis that intensive glycemic control soon after the diagnosis of type 1 diabetes could prevent the natural decline of endogenous insulin secretion. The impact of a short period of intensive insulin therapy has been studied in the past, but this gave inconsistent results.
The CLOuD study is the first study to investigate whether a long-term (2 years) treatment with a closed-loop system (here CamAPS FX) shortly after the diagnosis of type 1 diabetes, can save the C-peptide secretion (and thus the residual beta cell function). 101 adolescents (10-17 years) were randomized to closed-loop or a control group (without closed-loop) within 21 days of their diagnosis with type 1 diabetes. Their baseline HbA1c was 10.6%. After 2 years, as expected, glycemic control was clearly better in the closed-loop subjects (TIR 64%, HbA1c 6.9%), compared to the control group (TIR 49%, HbA1c 8.0%). And this despite the fact that a lot of technology was also used in the control group (43% wore a pump and 68% a CGM).
Unfortunately, however, there was no effect on the C-peptide. In both groups, the C-peptide decreased during the 2 years after diagnosis.
Sometimes the total insulin daily dose is also used as a parameter for endogenous insulin secretion. Again, no difference was noted between the total daily insulin dose in people on a closed-loop system (1.14 U/kg/d) compared to the control group (1.09 U/kg/d).
Thus, a prolonged period of closed-loop therapy after the diagnosis of type 1 diabetes in children and adolescents does not appear to stop the decline in C-peptide secretion. It could of course be that an even better glycemic control (eg normoglycemia) can slow down the deterioration of the C-peptide. Presumably, however, the decline of the beta cell is caused by other factors, for example the strength of the immune response.
It may not be the nicest message, but it is interesting to get to know the mechanism of type 1 diabetes better. However, this study shows that initiating a closed-loop immediately after diagnosis of type 1 diabetes is safe in children and adolescents, and provides much better glycemic control than insulin therapy without closed-loop technology.
2️⃣ Fiasp provides little added value with iLet insulin-only
The iLet closed-loop system from Beta Bionics was discussed extensively at the ADA. It was emphasized once again that the iLet has a completely new and different level of automation:
- The algorithm only uses the patient's weight for start-up, you don't have to enter any other parameters for insulin dosage
- After entering the weight, the closed-loop can start immediately (there is no warm-up period)
- The algorithm adapts autonomously and continuously to the patient
- The only thing you can adjust is the target value (normal, lower or higher), which you can set for different times of the day
- For meals, the patient only has to enter which meal he will eat (breakfast, lunch or dinner) and its size (normal, more than usual, less than usual or much less than usual). So no carbohydrates should be counted.
- All meal settings (carb ratio, insulin sensitivity, etc) are determined by the algorithm and cannot be adjusted
The iLet system therefore takes all calculations, and all decisions about insulin dosing, away from the patient, and also from the healthcare provider.
Beta Bionics completed a major clinical trial in December 2021 with their iLet insulin-only version: 440 adults and children (>6 years) were randomized to either the insulin-only iLet or regular diabetes care with a Dexcom G6. The results have not yet been published, but have already been discussed at the ATTD (see also my previous blog). The main result was that after 13 weeks the TIR (time in range) increased in the iLet group from 51% to 65% (in the control group the TIR only increased from 51% to 54%).
Several details of the study were discussed at the ADA:
- In the adults, the effect on the TIR was already visible from day 2 after the start of the iLet system. This is nice to see, especially because there was no run-in phase to get used to the system. People in the study (including those who previously only used insulin pens) were given a closed-loop iLet from day 1, based on their weight alone.
- It is striking that at the start of the study 31% of the people were already using a closed-loop system (in America only Control IQ or MiniMed 670G/770G). People in the control group were also allowed to continue using it. Then, if you look at a sub-analysis of the adults based on the technology they used at the start of the study, it makes sense that those who were not yet using a pump would benefit the most from a closed-loop system. But also in those who previously used a closed-loop, a limited improvement was seen in HbA1c. 60% of these people even reported that the iLet system was better or much better than their current closed-loop system!
- Most interesting was the comparison between the adults taking Novorapid or Humalog (n=103) and those taking Fiasp (n=113). The algorithm was not modified to use Fiasp. People taking Novorapid or Humalog had to fill their insulin reservoir themselves. Those on Fiasp could use prefilled ampoules. Overall, there was little difference between the 2 groups. There was no difference in HbA1c, mean glycemia, time <54 mg/dl, total insulin daily dose, severe hypoglycemia, ketoacidosis and patient reported outcomes (diabetes distress, hypoglycemia confidence, fear of hypoglycemia, treatment satisfaction). There was only a small difference in TIR (time in range): the TIR was 2% (29 minutes per day) higher in the Fiasp group compared to the Novorapid/Humalog group.
It has long been hoped that faster-acting insulins such as Fiasp will give better results in closed-loop systems. Fiasp has a slightly faster absorption and action than Novorapid (peak action comes 25 minutes faster), so that postprandial glycemia is slightly lower with Fiasp than with Novorapid (when using insulin pens).

In addition to this study with the iLet, it was also shown with the MiniMed 670G that Fiasp unfortunately does not give better results in closed-loop systems. So either the current "superfast" insulins aren't fast enough, or the algorithm doesn't work well with insulins with different pharmacokinetics. At the moment, in any case, the added value seems limited, if there is any.
When will the iLet come to us?
An FDA approval for the iLet insulin-only was requested this year (but could take another year), a CE approval has not been requested yet. So it will certainly take a few months/years before we can use the iLet with us. But it's coming, and it looks like a super easy system, with good results!

In the meantime, we are also waiting for the real iLet: the one with insulin and glucagon. The combination with glucagon will likely make the algorithm more aggressive, provide better glycemic control and give the option to omit meal announcements entirely. The study with the bihormonal iLet should have been started at the beginning of 2022, but appears to have been postponed to later this year (cfr press report from Zealand Pharma that supplies the dasiglucagon).
3️⃣ MARD update: Sensionics 365 9.8% and Simplera 10.2%

Data on the accuracy of the Eversense 365 and Simplera were shown for the first time at the ADA. Although you expect that the accuracy will increase with each next generation of sensors, this could not be demonstrated with these systems:
- The Eversense 365 is the successor to the Eversense E3, which received a CE label last month (16-6-2022) (it already had an FDA label). (Eversense E3 will be released in Germany, Italy, Spain, Netherlands, Poland, Switzerland, Norway and Sweden.) Like the Eversense E3, the Eversense 365 will require calibration 1x/d, but will last for 365 days in instead of 180 days. The ADA showed results from an Eversense E3 that they let sit for 365 days. The MARD of the first 180 days was 9.5%, and that of the following 180 days was 10.4%. It seems that the Eversense loses a bit of its accuracy if you wear it longer. The 365 day total MARD was still a good 9.8%, slightly higher than the "official" MARD from Eversense E3 (9.1%). The clinical studies with the real "Eversense 365" will start at the end of this year. Of course we are even more curious about their "Freedom System" where the transmitter on the skin finally becomes superfluous 😍. Eversense is also working on a 2nd generation of the "insertion tool" for healthcare providers, and integration of Eversense in closed-loop systems (for example with Beta Bionics).

- Simplera is the successor to the Guardian 4, and also does not require any calibrations. It is a sensor and transmitter in 1, which you can apply to the skin with 1 click, and which no longer requires overtape. Simplera will initially be launched on the market with a 7-day wear time, but a longer wear time is also being worked on. In this video Ali Daniaty also explains why the name Simplera was chosen instead of "Guardian 5". On the ADA there was a poster with the MARD data: the overall MARD was 10.2%, the same as the Guardian 4. This is slightly higher than the MARD of the Guardian 3 that is stated on the Medtronic website (9.6%). So Medtronic seems to have a little more trouble getting the MARD <10% in contrast to the other CGMs, but this difference will probably not be clinically significant. FDA and CE applications for Simplera are planned for this summer.
MARD stands for Mean Absolute Relative Difference, and is the most commonly used parameter to name and compare the accuracy of CGMs. However, it is not the best parameter and certainly does not say everything. The MARD value of a particular CGM system differs enormously from study to study, as also described in this article. In the absence of a better parameter/consensus, it is often assumed that a MARD of 10% is sufficient to speak of a reliable glycemic measurement at which insulin can be dosed. In general, we see that the MARD of the CGM systems is improving year over year and that the current CGMs are all meeting this MARD of 10%.

The current differences in the accuracy of the different CGMs are probably not clinically relevant. To choose one CGM system over another, it makes more sense to rely on other parameters, such as difference in sensor size, lifespan, warm-up time, cost, etc.
Although it was disappointing to see that CamAPS FX could not stop the decline in C-peptide, the CLOuD study did show that the initiation of a closed-loop system in diagnosis of type 1 diabetes is safe and provides superior glycemic results. The next step is to make the systems easier to use, and the iLet is on the right track! The CGMs are now also focusing primarily on ease of use. We are one step closer to a longer wear time for the Eversense, and a simpler application for the Medtronic CGMs.
I hope you have a chance to enjoy a beautiful summer - don't forget to keep an eye out for the exciting updates on diabetes technology!
Kind regards,