December update on closed-loop systems
Jan 04, 2022Hello early adaptors!
It's been a few weeks since you got an update on diabetes technology, so let's take a look at what happened last month in terms of closed-loop systems. There is a lot to report 🤗.
Besides one minor niggle that the FDA approval of the highly anticipated Omnipod 5 was again delayed, there was more good news than ever last month:
- Tandem presented 4 (!) new pumps
- ViCentra introduced their Diabeloop-Kaleido system
- Eoflow started a study on EOPatch X
- BetaBionics started a study for their bihormonal closed-loop system
- Medtrum apparently has a closed-loop system with their Nano pump in the pipeline
- and the Sigi patch pump received a "Breaktrough Device Designation" from the FDA
A great start to a new year.
🎉🎉🎉Happy New Year everyone!
Get Access To Updated Diabetes Technology Courses
⭐ Omnipod 5
Shacey Petrovic, the (female 😊) CEO of Omnipod, announced at a Nasdaq meeting on December 3th, that the FDA approval of Omnipod 5 has been postponed to the first quarter of 2022. Fortunately, she emphasized that Omnipod 5 will be offered at the same price as Omnipod DASH, and that the start-up/onboarding is very simple.
They are also working on the expansion in international markets (however, still no timeline for Omnipod 5 in Europe 😥), indication in children 2-5 years, type 2 diabetes and an app for iOS (cfr. Omnipod 5 is currently only available for Android).

⭐ Tandem
Tandem organized a virtual R&D day for the first time on December 6th, you can download their presentation here. With their new product launches (both hardware and software), they aim to triple their number of users (from 300,000 to 1 million) by 2027!
Over the next 5 years, Tandem will launch 4 (!) new pumps (+ an extended wear infusion set):
- the 50% smaller Mobi pump with phone-control (this used to be called t:sport, and won't be more expensive than the t:slim X2 pump, if only because it's 20% cheaper to make - just like the accompanying reservoir)
- the t:slim X3 pump (with better technology, longer battery life and possibility of wireless updates)
- the Mobi: tubeless pump (this is the Mobi pump that you can place on a pump base)
- and finally also a real disposable patch pump!






They emphasized that >200,000 people now use Control IQ and referred to the good results in this real world study with 9,500 users.
Their next generation closed loop (Control IQ 2.0?) will contain more personalization (such as more choice for the target value, and new bolus and sport options) and be easier to use (thanks to adaptation and smart alarms, among other things). They do not mention any goals to improve the TIR as we see with other companies.
They also want to extend the indication to children 2-5 years, people with type 2 diabetes and use with other insulins (ultra fast-acting insulin, concentrated insulin, and biosimilars).
For people in the US, the t:connect Mobile app is already available, with which you can track the data from your pump (analogous to the MiniMed Mobile app). They expect FDA approval for their phone-bolus option with the t:slim X2 pump in early 2022, and are developing an app with full phone control for their Mobi pump (which will also allow for wireless software updates).
In addition to the Dexcom G6, the algorithm will also be made available for integration with Dexcom G7 for existing and new users, and in the future Libre (once FDA clears it as iCGM). No timeline was given for this.
The software of t:connect will be updated to Tandem Source. With this they want to offer more efficient data visualization, with automatic trend recognition and advice in the long run. By automatically adjusting the pump settings, they hope to achieve even better results, and also to reach patients who do not have access to an endocrinologist (cfr in many regions there are not enough endocrinologists and people with (type 1) diabetes are followed by the general practitioner).

⭐ Diabeloop-Kaleido
Vicentra (>The Netherlands, Utrecht), the producer of Kaleidopumps, has raised 65 million euros in capital to scale up the production of their Kaleidopump. Kaleido pumps were temporarily on the market in the Netherlands, but due to production problems, the users had to stop in 2020. They will be able to restart from this year. Kaleido would recently also be on the market in France, becoming available this summer in Germany and England, and in more European countries in 2023. An FDA filing is also underway.
The Kaleido pump is unique because it is small and light, and is available in many colors. You can choose between a short infusion set or one of 30 cm.
The pump is controlled from a remote control similar to an earlier iPOD, or via the Diabeloop handset which also includes the DBLG1 algorithm and can connect to the Dexcom G6. The Kaleido-Diabeloop system could therefore become the first AID system with a semi-patch pump in Europe (small disclaimer: this is speculation).

⭐ Eopanceas
EOFlow (>South Korea), the producer of the EOPatch pump, is at cruising speed! After a sales agreement in China, last month they signed a major contract with a distributor in Indonesia, and won 3 tenders in Italy (by Menarini, who will distribute the pumps in Europe).

In addition to their EOPatch pump (which is very similar to the Omnipod cfr previous blog post), they are also developing a closed-loop system with an integrated CGM: the EOPancreas. For this they already received a Breaktrough Device Designation from the FDA in 2019.
This month they will start a study with their "EOPatch X" in 100 people with type 1 diabetes at 9 university hospitals in Korea. The EOPatch X is a hybrid closed-loop system consisting of 2 parts: the EOPatch pump with an algorithm on the pump, and a CGM (not specified). With this study, they hope to have enough data by the end of this year to commercialize their EOPatch X in 2023.
⭐ BetaBionics
We mainly know BetaBionics for their iLet Bionic Pancreas that they are developing. This is a bihormonal closed-loop system, in which there are both insulin and glucagon in one pump. The user only has to enter his weight, so no meals or sports, and no basal settings for insulin. They received a Breaktrough Device Designation from the FDA for this in 2019. The first results looked promising (TIR up to 79% in the bihormonal iLet).

The pivotal study of the insulin-only hybrid closed-loop system ended last month. This randomized controlled multicenter study enrolled 440 adults and children >6 years with type 1 diabetes. The results will be discussed at the ATTD 2022 😀.
A new pivotal study with the bihormonal complete closed-loop system was also started in December 2021. The goal is to include approximately 700 adults and children >6 years of age with type 1 diabetes. The study will use Fiasp and Dasiglucagon.
Until recently we assumed that bihormonal closed-loop systems were the logical next step to close the loop. We are now hearing more and more arguments that insulin-only closed-loop systems may be "good enough". So let's see if bihormonal closed-loop systems will ever get into our hands, as they presumably will be much more expensive.
⭐ Touchcare Nano system
Medtrum Technologies (>China) is the distributor of the Touchcare Nano insulin patch pump and the Touchcare Nano CGM. Their patch pump is a lot smaller and lighter than the patch pump from Equil and the Omnipod and yet contains the same amount of insulin (200 U).
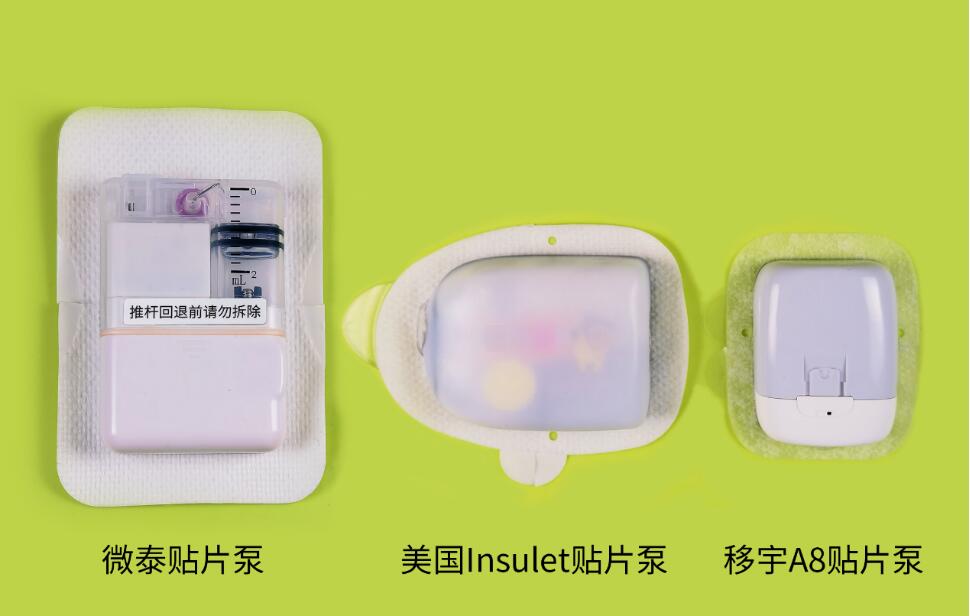
It is operated via a remote control or with the EasyPatch app. If you link the pump to the corresponding CGM, you can activate a stop-before-low function on the pump,
The Touchcare Nano A7+ system is available in the Netherlands (although the version shown here looks different), England, France, Germany, Denmark, Finland and Sweden.
At the JDRF Fusion session of December 13th, the representative said that a hybrid closed-loop system is also coming "soon", namely the Touchcare Nano System (Nano pump + Nano CGM + APGO algorithm). In this article it is claimed that there will also be a new generation A8 closed-loop system, where entering carbs for meals will no longer be necessary.
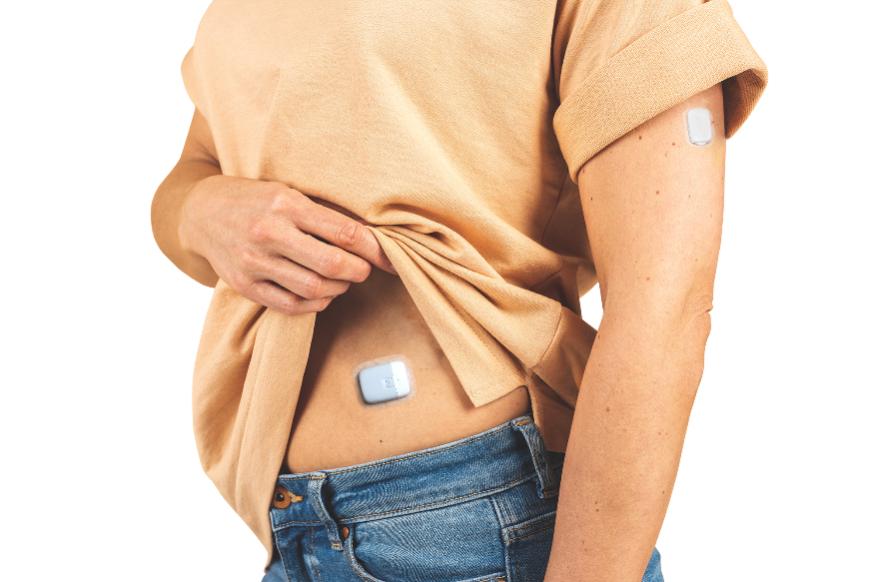
⭐ Sigi patchpomp
AMF Medical (>Switzerland) has received a "Breaktrough Device Designation" from the FDA on November 30th for its Sigi modular patch pump with prefilled insulin cartidges. The pump is smaller than the Omnipod, and would be easier to use. It is phone-controlled, has a (patented) high-precision insulin delivery and fast occlusion detection. Clinical studies will start in the 2nd half of 2022, and the pump could hit the market in 2024-2025.
All these novelties certainly make me look forward to the coming years 😍.
In any case, I wish you an inspiring 2022 with many peak experiences and that all your wishes may become reality.
Kind regards,