Top 10 of the ATTD - part 2
May 25, 2022Join our free courses on CGMs, pumps, and AID systems
So much information was shared at the ATTD congress last month that I couldn't get it in 1 blog post. This is the 2nd part of the 10 most important updates on diabetes technology from Barcelona. Wondering if there's anything you'll be using soon? Then read on quickly!
- SGLT2-inhibitors increase TIR in Tandem Control IQ
- Diabeloop develops the DBL4 pen with Mallya
- Sanofi Solo Smart button
- No news from Roche, Inreda, Eoflow, Medtrum
- International consensus on automated insulin systems (AID)
Get Access To Updated Diabetes Technology Courses
6. SGLT2-inhibitors increase TIR in Control IQ

The use of SGLT2-initiators as an adjuvant treatment (ie on top of insulin) in people with type 1 diabetes is controversial. Although it improves glycemia and lowers the risk of renal insufficiency and cardiovascular disease (as in people with type 2 diabetes), it is not approved by EMA/FDA in people with type 1 diabetes because of the excessive risk of ketoacidosis.
It has been argued for some time that adding an SGLT2-inhibitor could improve the performance of closed-loop systems and reduce the need for carbohydrate counting. The recently published CiQ-SGLT2 study was discussed at the ATTD, in which an SGLT2-inhibitor was studied for the first time (over a longer period of time) in combination with a closed-loop system. In this study, 35 adults with type 1 diabetes were randomized to receive or not to receive empagliflozin 5 mg/d, after which they wore a Tandem pump with Control IQ (=closed-loop) for 4 weeks, and then for 2 weeks with Basal IQ (stop-before-low algorithm). Both in Control-IQ and Basal-IQ, the daytime TIR (time-in-range) was - expectedly - higher in the people taking empagliflozin, without increasing the risk of hypoglycemia:
- Control IQ: Daytime TIR 81% with empagliflozin vs 71% without empagliflozin
- Basal IQ: Daytime TIR 80% with empagliflozin vs 63% without empagliflozin
Despite the low dose of empagliflozin, an increased risk of ketoacidosis was noted: in the empagliflozin group, 1 person developed ketoacidosis requiring hospitalization due to a pump failure. Also notable is the much higher degree of ketosis without ketoacidosis in people taking empagliflozin (13 out of 18 people on Control IQ!). This may also have to do with the fact that the people on empagliflozin had to check their ketones much more often in the study, but still! This case study from Leuven also showed that ketoacidosis with an SGLT2-inhibitor is indeed possible, even in a closed-loop system.
Monitoring of ketones and proper patient education remains clearly necessary if an SGLT2-inhibitor is associated in someone with type 1 diabetes on a closed-loop system. Going forward, continuous ketone monitors may increase the safety of SGLT2-inhibitors. And maybe we can then increase the TIR by another 10%, just like in this study, or make carbohydrate counting superfluous 😀.
7. Diabeloop develops DBL-4pen with Mallya

Diabeloop announced on 4/12/2022 that they were partnering with Mallya from Biocorp (and Dexcom G6) for their DBL-4pen. This could be the first smart insulin pen to provide real-time advice for both bolus insulin and basal insulin. Within a few weeks, the first studies with this system would start in France. In the future, they would also like to work with other smart insulin pens. With the DBL-4pen they want to achieve 80% of the results of a closed-loop system (with an insulin pump) at 30% of the cost. I'm curious!
Other updates of Diabeloop:
- About 7,000 people are already using Diabeloop in combination with the Accu-Check Insight or the Kaleidopump.
- Real-world results from 974 Diabeloop users in Germany (September-December 2021): TIR increased from 54.% to 73%, TBR 0.9%, <54 mg/dl 0.1%.
- Diabeloop also develops a fully closed-loop system (so without the need to enter sports and/or meals), with which they want to achieve a TIR of >90%! To do this, they want to include additional sensors via a smartwatch (such as heart rate variability and an accelerometer), for automatic detection of physical activity and meals. A bit in the same vein as Medtronic, which will also work with meal detection via a smartwatch. You can also find the updates from Diabeloop in this YouTube video.
8. Sanofi SoloSmart button

Mallya is Biocorp's smart insulin pen and normally consists of 2 pieces. There is a separate version for the Flexpen, Kwikpen and Solostar, and one is also being developed for the FlexTouch. The ATTD also had the latest version of Mallya, which consists of only 1 piece (cfr photo). This small attachment would have the same functionalities as the larger version. It is currently only developed for Sanofi and will be called the SoloSmart button. It would come on the market "soon" and fit on all Solostar pens from Sanofi.
This SoloSmart button looks suspiciously like Lilly's Tempo Smart Button, which in turn will only fit Lilly's Tempo pens. The CE label for this was expected at the end of last year, but is now expected - together with an FDA label - in the 2nd half of 2022. Novo Nordisk is not sitting still either and is developing Dialoq (a smart insulin pen cap), next to their Novopen 6 (a solid smart insulin pen). And so all the major insulin companies have a smart insulin pen cap for their most used disposable pens in the pipeline. Hopefully they will include this with their insulin for free, because unfortunately there is no reimbursement for smart insulin pens and/or caps yet...
9. No news from Roche, Inreda, Eoflow, Medtrum
The ATTD is the largest symposium on diabetes technology and there is also always a big "tech fair" where all the major companies are normally present. Strange that Roche, one of the major insulin pump manufacturers in Europe, was not there. 2 years ago they were there with their Solo pump, but this year there was no one to ask critical questions... The Solo pump has been available in Belgium for a few months and it would even be the intention that this pump completely replaces the Insight pump in a few years. Like the Insight pump, the Solo pump can be used as a closed-loop system via a connection to Diabeloop and Dexcom G6.
Things I was looking for but couldn't find at ATTD:
- Inreda: In their newsletter this summer, there was mention of a follow-up study with 75 people, and they are also known to be working on a smaller device. For the time being, however, little news and no Inreda stand at the ATTD. In this article of 19-5-2022, Robin Knoops says that after the summer of 2022, a study will start with 240 people in 10 hospitals in the Netherlands (120 people receive Inreda and 120 people enter the control group). He expects Inreda's AP® to become widely available in 2024!
- EoFlow: This South Korean insulin pump manufacturer has already mentioned a few times that they will develop a closed-loop system in the short term, however at the Menarini booth (which distributes these pumps under the name GlucoMen Day Pump) they couldn't do much for me to tell. This update did appear on their site on 20-5-2022: the first patient would have started using the EoPatch X in a study context. EoPatch X will be tested on 104 people with type 1 diabetes in 9 hospitals in Korea. This is a closed-loop system with the EoPatch insulin pump, "one" CGM and "one" algorithm. I'm especially curious about the last two. Furthermore, I also heard at MedTech JRDF that the Narsha app (with which you can control the pump) is expected this summer in Belgium and the Netherlands, and that at that time the GlucoMen Day pump will also be available (in manual mode). The first patch pump controlled by your mobile phone (in our region).
- Medtrum: This Chinese insulin pump manufacturer has also mentioned several times that they are working on a closed-loop system. This would consist of their TouchCare Nano insulin pump (the smallest insulin pump on the market), the TouchCare Nano CGM and a modified APGO algorithm. Different versions of their insulin pump were on display at their ATTD booth, but they couldn't say much more about a possible closed-loop system.
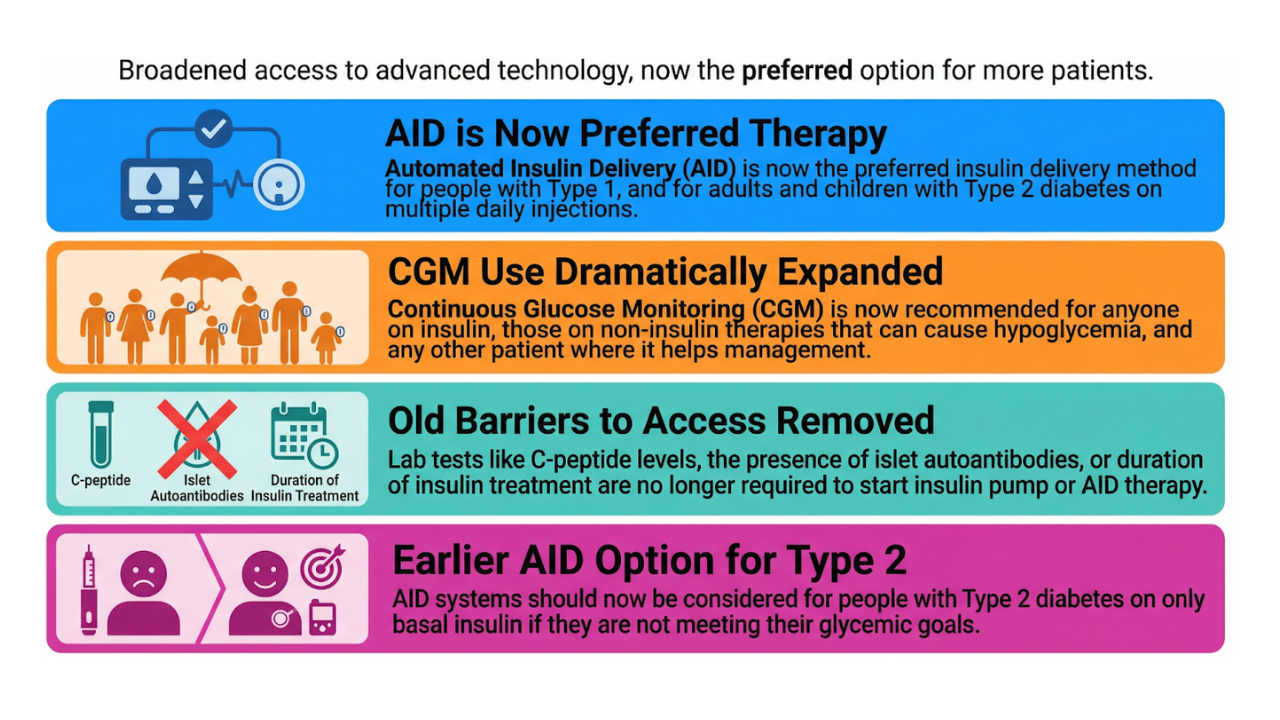
10. Internationale consensus over closed-loop systemen
So much new technology and so many opinions on how to use it and for whom...so time to make a consensus! Dr Moshe (Israel), Dr Nimri (Israel) and Dr Battelino (Slovenia) hosted "The International Consensus Recommendations for the Use of Automated Insulin Delivery Technologies in Clinical Practice" last year with more than 40 "key opinion leaders" and came to some decisions, including:
- Extensive randomized studies have shown that closed-loop systems increase TIR by 9-16% and decrease HbA1c by 0.3-0.5%, with no change or decrease in TBR (time-below-range). Real-world data supports these findings time and again. Glycemia improved across all age groups, independent of duration of diabetes, prior mode of insulin administration, or baseline HbA1c. The lower the TIR at the start, the higher the TIR will rise. Closed-loop systems should therefore be recommended for anyone with type 1 diabetes. You may consider recommending it for children <7 years and adults >65 years with type 1 diabetes. There is less evidence for closed-loop systems in people with type 1 diabetes who are pregnant, in people with type 2 diabetes, diabetes associated with cystic fibrosis or after pancreatectomy for now (but studies are ongoing).
- There is not enough data to initiate closed-loop systems from the start of the diagnosis of type 1 diabetes, but studies will probably show a benefit in terms of long-term glycemic control and also acceptability of the devices.
- A list has been drawn up for structured education that is best given at the start of a closed-loop system, but also during its follow-up.
- And finally, a strong recommendation for insurance companies and governments: to make closed-loop systems available to all people with type 1 diabetes who want to use them, along with the necessary education and support with the start and follow-up.
I look forward to the publication of this consensus meeting! I certainly already know some key opinion leaders who will be happy to present this summary to their insurance company 😁.
These were my 10 highlights from the ATTD on diabetes technology. The first 5 updates can be found here.
If you would like more information on any of these topics, or if you have any news of your own about diabetes technology, be sure to let us know! This way I know better what interests you most and I also learn more. And if you know anyone else who might be interested in this kind of information, feel free to send them the link to this blog post!
Kind regards,

Join our free courses on CGMs, pumps, and AID systems