Virtual Diabetes Technology Meeting 2022 (english)
Dec 06, 2022
This year's 22nd annual Diabetes Technology Meeting (DTM) took place virtually on November 3-5, and showcased some exciting updates and new products.
This blog highlights some of the highlights:
- Prospective study validates Tandem Control IQ in people with type 2 diabetes
- Inhalable insulin in combination with AID systems
- Proof of concept combined infusion set and glucose sensor SynerG
- Accuracy of Abbott's Continuous Ketone Monitor
- Prototype continuous glucose measurement in urine in diapers
The DTM is organized each year by the Diabetes Technology Society.
This association has existed since 2001 and was founded by Dr. David Klonoff with 1 goal: to promote the development and use of diabetes technology!
Get Access To Updated Diabetes Technology Courses
Read on below for more details on the highlights of the DTM!
1. Prospective study validates Tandem Control IQ in people with type 2 diabetes
Tandem Control IQ is the most widely used closed-loop system, with more than 270,000 users worldwide (most of them in America).
This Automated Insulin Delivery (AID) system is not only used by people with type 1 diabetes (DM1), but also by people with type 2 diabetes (DM2) - although Control IQ does not yet officially have this indication.
In several real-world studies we already saw that Tandem Control IQ works well in these people with DM2:
- Forlenza et al. DTT 2022
- Retrospective analysis of 5575 people with diabetes, of which 500 people with DM2.
- In people with DM2, the Time In Range (TIR) increased after 1 month from 64% to 72% (+8%).
- Breton et al. DTT 2021
- Retrospective analysis of 9451 people with diabetes, of which 378 people with DM2.
- In people with DM2, the TIR increased from 70% to 78% (+8%) after 12 months.
On the DTM, a first prospective analysis of Tandem Control IQ was shown in 30 adults with DM2.
- In these 30 adults, 15 people used basal-bolus insulin, 2 people used an insulin pump, and 13 people used only basal insulin.
- In addition to insulin, many of these people also used SGLT2 inhibitors and GLP-1 analogues.
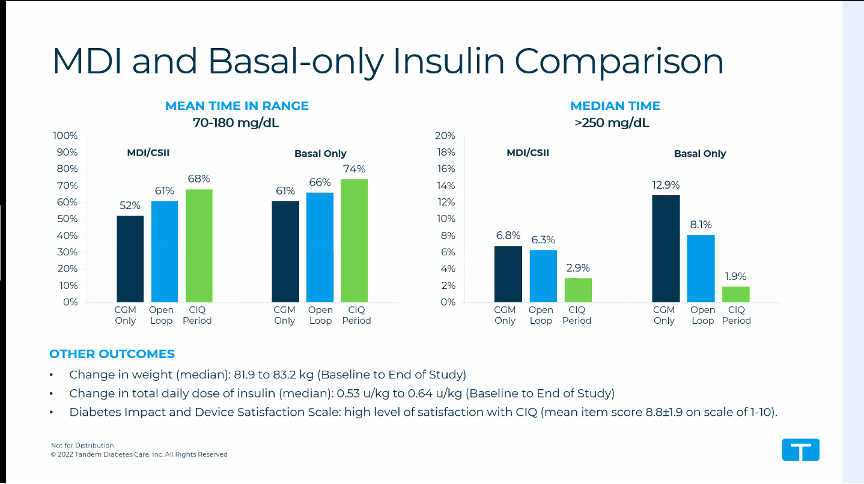
After 6 weeks with the Tandem Control IQ, the TIR increased significantly as expected:
- in the total group the TIR increased from 56% to 71% (+15%)
- in the group that used basal-bolus insulin beforehand, the TIR increased from 52% to 68% (+16%)
- in the group that used only basal insulin beforehand, the TIR increased from 61% to 74% (+13%)
The TBR (<70 mg/dL) did not change, but no numbers were shown.
After 6 weeks, on average slightly more insulin was used in closed-loop (0.64 U/kg instead of 0.53 U/kg), and weight increased slightly (83.2 kg instead of 81.1 kg), but this does not seem to be a clinically important increase.
These are important parameters in DM2, because - even more so than in DM1 - we do not want people to gain weight from the extra insulin they may receive in closed-loop.
Other closed-loop systems in type 2 diabetes
These results are similar to the prospective study of Omnipod 5 in people with type 2 diabetes discussed at the ATTD.
- In their study, 24 people with type 2 diabetes were given an Omnipod 5 for 8 weeks:
- In the 12 people on basal-bolus insulin, the TIR increased from 46.5 to 60.5% (+14%)
- In the 12 people on basal insulin alone, the TIR increased from 32.2% to 56.6% (+24%)
- In this study, the daily insulin dose fell from 92 to 63 U in the basal-bolus group, and the insulin daily dose increased from 30 to 42 U in the basal-only group.
- However, in both groups it was shown that there was no effect on weight (after 8 weeks).
The CamAPS group also published a lot of research on type 2 diabetes this year.
- Results of CamAPS HX (a fully closed-loop system) were shown at EASD in 26 people with type 2 diabetes on basal-bolus insulin (without CGM).
- After 4 months, the TIR increased from 32% to 66% (+34%) without causing hypoglycaemia.
Decision
Closed-loop therapy appears to be safe and effective in DM2, possibly even more so than in DM1.
Although AID systems are already often used in people with DM2 (especially in America), the indication is not yet official.
The first steps have now been taken with small prospective studies, for example this study with Tandem Control IQ.
The next step is larger "pivotal trials", after which the official indication for DM2 can be requested from the FDA and Europe.
The manufacturers of AID systems are therefore - rightly so - trying to expand their market to people with DM2 on insulin.
Of course, it is not certain whether this expensive technology will ever be reimbursed for this large population.
2. Inhalable insulin in combination with AID systems

We do not know it, but in America inhalable insulin is sometimes used.
Afrezza (>Mannkind) has been on the market there since 2014, although commercialization has been difficult so far.
Fast in, fast out
The unique thing about inhalable insulin is that it starts working very quickly and stops working very quickly.
Ideal for catching a meal/snack.
.png)
Afrezza is available in 4 U, 8 U or 12 U, but these units do not match the units of regular insulin!
- 4 E Afrezza is approximately equivalent to 2.5 E Novorapid,
- and 8 E Afrezza roughly corresponds to 4 E Novorapid.
Attention: Afrezza is only approved for people >18 years old, and should not be used by people with asthma/COPD or by smokers.
Side effects include hypoglycemia, cough, sore throat and laryngitis.
Afrezza in combination with an AID system
Afrezza is super fast-acting insulin, which you can use in combination with slow-acting insulin injections or with an insulin pump.
A study by Afrezza with an AID system was shown at the DTM 2022.

In this prospective study, 26 people were randomized to:
- Afrezza + closed loop
- or rapid-acting insulin + AID system (no further specification).
The people who used inhalable insulin had less postprandial hyperglycaemia than the people who administered an insulin bolus with their AID system.
Only 1 asymptomatic hypoglycemia was noted (54-70 mg/dl).
(It is not really clear which AID system was involved, and whether there was any effect on the overall TIR.)
Decision
This study shows that inhalable insulin can be safely combined with an AID system.
And this option is also listed by some major diabetes centers in America.
Afrezza is not available in Europe, so this is not an issue for us for the time being.
3. Proof of concept combined infusion set and glucose sensor SynerG

People who wear an AID system currently still have to wear 2 devices on their body:
- a catheter pump or patch pump infusion set
- and a glucose sensor.
By combining these 2, people will be able to use an AID system with only 1 device attached to their body.
Pacific Diabetes Technologies has been actively working on such a combined infusion set and glucose sensor since 2014.
Their prototype is called SynerG.

A "feasibility study" was shown at the DTM where 8 people with DM1 received a MiniMed 780G pump with their SynerG infusion set, as well as a Dexcom G6 sensor.
The people had to eat 2 standardized meals (study period) after a run-in period of 48 hours.
- The glycemic sensor in their SynerG prototype had a MARD of 13.9%.
- Total insulin daily dose and TIR were identical between study period and run-in time.
So the SynerG system seems to be working.
Although there were no clear clinical implications here, a MARD of ≤ 10% is normally required for a sensor to be used in a closed loop.
The decision is therefore that they will continue to work on improving the accuracy of their sensor.

Medtronic is also conducting research into a combined infusion set and sensor, the Medtronic Duo Extended Set.
- At ADA 2021 we received an update on a preclinical study in pigs.
- We know that studies have started in humans, but we haven't received an update since then.
4. Accuracy of Abbott's Continuous Ketone Monitor
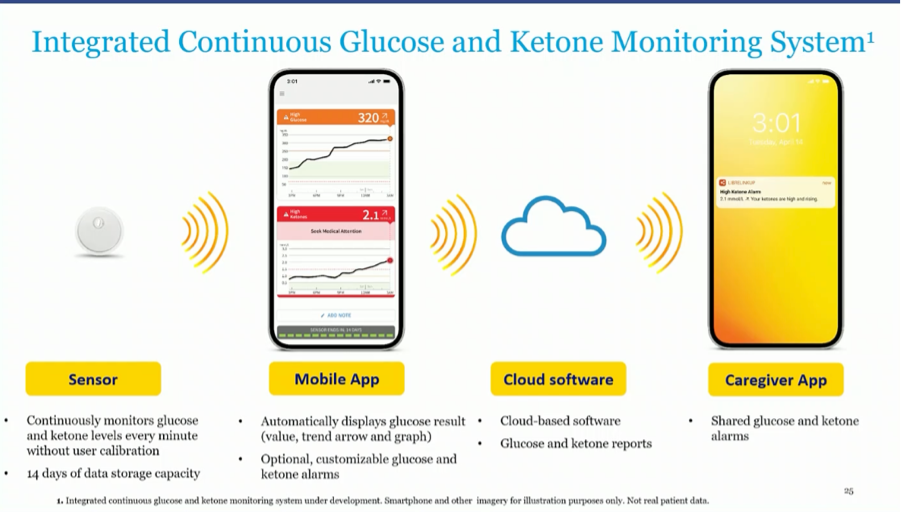
The image above has already been shown at EASD and shows that Abbott is working on a combined glucose and ketone monitor.
The ketone measurements will take place 1x/minute and no calibration is required.
As a reminder, ketones (in the form of beta-hydroxybutyrate) in the blood/capillary are usually measured with these cut-off values:
- <0.6 mmol/l: normal
- 0.6-1.5 mmol/l: ketones present in the blood
- 1.6-3.0 mmol/l: impending ketoacidosis
- >3.0 mmol/l: probable ketoacidosis
A study was already published in 2021 that the Libre2 sensor was able to measure beta-hydroxybutyrate
- in 12 healthy people on a low-carbohydrate diet.
- Sensor readings were compared to capillary blood ketone strips (Precision Xtra test strips)
- The sensor was calibrated once with these ketone strips.
- The Mean Absolute Difference (MAD) of the sensor was 0.1 mmol/L for ketones <1.5 mmol/L.
- The Mean Absolute Relative Difference (MARD) of the sensor was 14.4% for ketones >1.5 mmol/l.
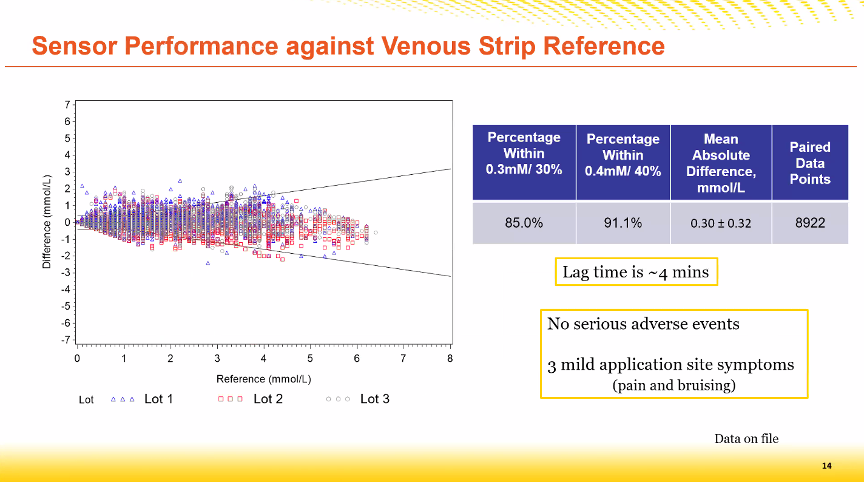
A follow-up study was now presented at the DTM 2022 in which beta-hydroxybutyrate was measured with the Libre2 sensor
- in 36 healthy people following a normal diet, but taking exogenous ketones.
- The sensor readings were again compared to their capillary blood ketone strips (Precision Xtra test strips).
- This time the sensor was not calibrated anymore.
- The Mean Absolute Difference (MAD) was 0.3 mmol/l for all measured ketone values.
- A delay of 4 minutes was seen between the capillary result (test strips) and the interstitial result (sensor).
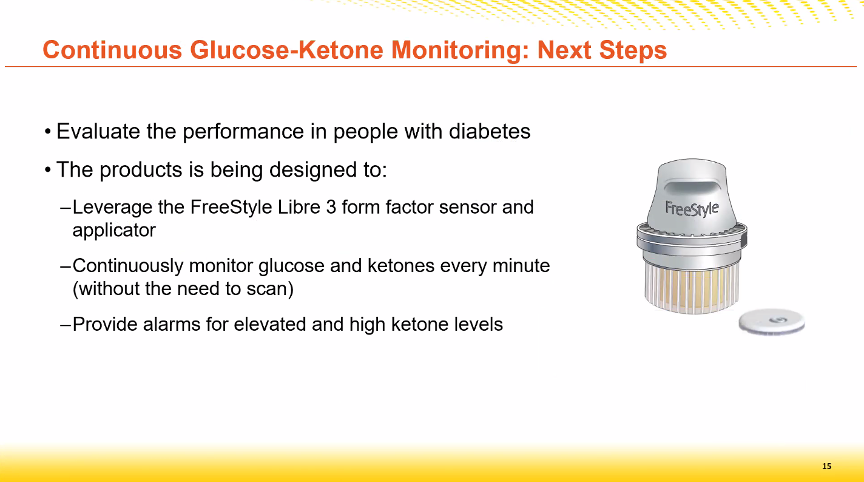
In the next step
- this ketone sensor will be integrated into a Libre3 sensor,
- it will no longer be necessary to scan the sensor
- and alarms for high ketones will be integrated.
A more large-scale "pivotal" study in people with diabetes is planned in 2023.
Abbott also plans to integrate this combined glucose and ketone monitor with AID systems.
This will probably make it possible to associate SGLT2 inhibitors with AID systems.
We know that this can further improve glycemic control (cfr CiQ-SGLT2 study: TIR from 71 to 81%).
5. Continuous glucose monitoring in urine in diapers
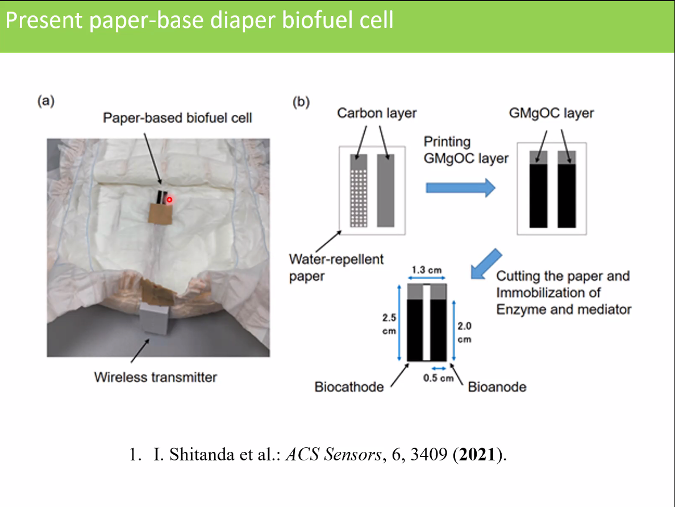
And finally a piece of news from Japan's Tokyo University: a prototype of a glucose sensor in diapers was developed there to facilitate care for the elderly.
- The sensor continuously measures the glucose in urine and sends it to an app on a mobile phone via Bluetooth.
- The system does not have a battery, but generates electricity from the urinary glucose via a "biofuel cell".
- The system mainly consists of a type of paper, which makes it cheap to make and has little impact on the environment.

Although we do not yet know the accuracy (MARD) of this glucose sensor, the product is so innovative that it was worth mentioning it here.
The 22nd Virtual Diabetes Technology Meeting (DTM) was a success, even though it was only virtual this year.
During this meeting, many innovative studies and technological advances in diabetes care were highlighted.
Some of these highlights include new developments in closed-loop systems for type 2 diabetes, inhalable insulin in combination with closed-loop systems, combined infusion sets and glucose sensors, continuous ketone monitors and even continuous glucose monitors in diapers.
This technological advancement proves that even if we have to do things differently for now, there is still a lot of progress being made.
We are excited to see what else 2022 has in store for us and will be sure to keep you up to date with all the latest news from the world of diabetes technology.
Kind regards,