#ATTD2023: updates on CGM
Mar 17, 2023
Several new applications of glucose sensors were discussed at the ATTD congress. It is clear that the popularity of continuous glucose monitoring (CGM) is growing, as more and more people with diabetes benefit from it. There was even a special session dedicated to diabetes and CGM at the innovative SXSW Festival in 2023, underlining the growing interest in this technology.
- CGM in people with type 2 diabetes who do not use insulin
- CGM to predict risk of type 1 diabetes
- CGM to predict gestational diabetes
- SXSW2023: CGM momentum is building
“What will happen with CGM in diabetes? I think it will not be five years before we look back and say, ‘do you remember the day we pricked our finger?’ It’s like the revolution of pens in Europe. Do you remember when we had syringes? I think it will be the same with CGM and that it will go very fast." - prof Chantal Mathieu, introductory lecture ATTD 2023 "The new face of diabetes"
Read on below for updates.
1. CGM in people with type 2 diabetes not using insulin
With major guidelines recommending CGM in all people with diabetes using insulin, the next question is:
what is the evidence for CGM in people with type 2 diabetes who do not use insulin?
Dr Bergenstal indicated that there is probably not yet enough scientific evidence to convince health insurers to reimburse CGM in people with type 2 diabetes without insulin, but that studies are already in place, and are in full swing. The following data was cited, among others:
- IMMEDIATE RCT (Aronson et al. Diabetes Obes Metab 2022): n=116 people with type 2 diabetes without insulin and HbA1c ≥ 7.5% were randomised to having or not having a Libre sensor. After 16 weeks, HbA1c dropped by 0.3% and TIR increased by 10% more compared with the control group.
- Data from nearly 6,000 Level2 users ("Undo Your Type 2"), a membership programme where people with type 2 diabetes receive a Dexcom G6. The HbA1c dropped from 7.7% to 7.0%
- Data from WellDoc BleuStar, a similar membership programme. In 91 people with type 2 diabetes without insulin who received a CGM, after 6 months HbA1c dropped from 8.8% to 7.7%, and TIR increased from 25% to 55%.
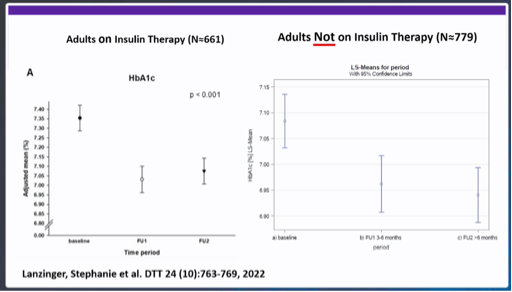
- Lanzinger et al. DTT 2022: evaluation of a German data registry with n=4434 adults with type 1 and type 2 diabetes who started CGM. Looking only at those without insulin (n=779), there was a clear decrease in HbA1c after starting CGM.
Small study shows good results of Dexcom G6 in people with type 2 diabetes without insulin
Dr Thomas Grace (Ohio, US) also showed promising initial results of CGM as first-line treatment for ALL people with type 2 diabetes.
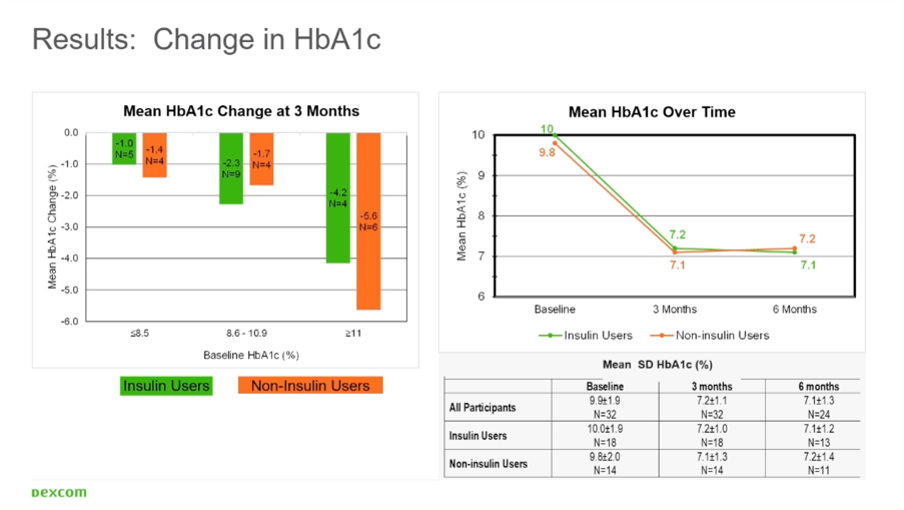
In his study, people with type 2 diabetes and an HbA1c >7.4% were given a Dexcom G6 sensor for 6 months:
- In the people who did not use insulin (n=11), the HbA1c dropped from 9.8% to 7.2%.
- In those who did use insulin (n=13), the HbA1c dropped from 10.0% to 7.1%.
Thus, the results were identical in those who used or did not use insulin.
These people were simply followed up with their general practitioner (GP), and received remarkably little education around the use of the CGM.
- At start-up, users were given a basic explanation of how a sensor works and how to apply it. After that, they had to figure it out on their own.
- The GPs themselves received no education on CGM use or interpretation.
During the study, it became clear that people with diabetes were able to use and interpret CGM without much supervision from healthcare providers. GPs were also familiar with Dexcom Clarity fairly quickly.
"CGM is first-line treatment for people with diabetes" - Dr Thomas Grace
(This video comes from the very interesting education platform Managed Care CGM.com)
It is clear that the conversations around CGM use in type 2 diabetes are changing. At ATTD2023, Dr Grace recommended that CGM be considered as first-line therapy for all people with diabetes.
Although there is a difference in the needs in different groups of people with diabetes, CGM is effective in everyone with diabetes:
- In people using intensive insulin regimens, CGM serves as a safety tool, to prevent hypoglycaemia.
- In people not using intensive insulin, CGM serves as a tool to change your behaviour.

(figuur > Comprehensive Type 2 Diabetes Management Algorithm 2020 AACE)
The majority of guidelines in diabetes algorithms (cfr figure) are about HbA1c and adjusting medication, but at the very top it says that the first-line treatment is lifestyle therapy and "ongoing glucose monitoring". With continuous feedback from a CGM, lifestyle therapy can be much more effective.
Other sessions also argued that we should no longer speak of CGM, but of CGMT or CGM-Therapy because CGM does more than monitor glycaemia. In fact, CGM is also a means of treating your diabetes.
2. CGM to predict risk of type 1 diabetes

(Figure derived from Powers et al. J Clin Invest 2021)
Since teplizumab was approved in America (17-11-2022), more and more questions arise about how we would best screen for type 1 diabetes.
Indeed, if we can find people at increased risk of type 1 diabetes, we could potentially slow the progression to "true" type 1 diabetes with teplizumab.
Note: Currently, teplizumab is only available in America.
Here you can find a nice testimonial from one of the first people treated with Tzield outside of study settings:
In Europe, teplizumab is only available in study trials, as it does not yet have a CE label.
Last week, Sanofi bought Provention Bio, so in the future Tzield will be distributed by Sanofi.
How can you screen for type 1 diabetes?
Screening for type 1 diabetes is done routinely by determining 4 autoantibodies in your blood:
- against insulin,
- against glutamate decarboxulase (GAD65),
- against islet antigen (IA-2),
- and against zinc transporter 8 (ZnT8).
As soon as you have more than 2 autoantibodies, you have type 1 diabetes in 1 of these 3 stages:
- Stage 1 = ≥2 autoantibodies + normal glycaemia
- Stage 2 = ≥2 autoantibodies + slightly elevated glycaemia without symptoms
- Stage 3 = ≥2 autoantibodies + elevated glycaemia (fasting ≥126 mg/dl or 7 mmol/l, 2 hours after meal ≥200 mg/dl or 11.1 mmol/l) = "clinical/real type 1 diabetes"
If you are in stage 1 or stage 2, you have almost a 100% chance of ever progressing to stage 3, even if you do not have type 1 diabetes in your family.
- People in stage 1 have 44% chance of progressing to stage 3 in the next 5 years, 70% in the next 10 years and 84% chance in the next 15 years.
- People in stage 2 have a 74% chance of progressing to stage 3 in the next 4 years.
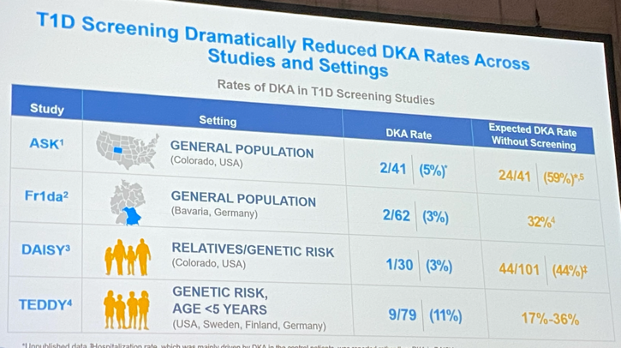
The purpose of screening is 2-fold:
- first, to reduce the risk of reporting with diabetic ketoacidosis and hospitalisation (cfr also ISPAD Guidelines 2022),
- but also to find those at increased risk of type 1 diabetes in order to include them in studies or treat them.
"To screen or not to screen, that's the question." (dr. Phillip)
At the ATTD, Dr Moshe Phillip (Israel) stated that we urgently need more guidelines on:
- when should we screen,
- how should we screen,
- what should be done with the results,
- and how people at increased risk of type 1 diabetes should be followed up.
Usually, with people with stage 1 and stage 2 of type 1 diabetes get an OGTT (Oral Glucose Tolerance Test) every 6 months.
However, this is a stressful test that requires you to stay sober for 8 hours, have your blood drawn, drink a sugary drink, and then have your blood drawn again 1 and 2 hours later.
Especially for children, who are at the highest risk, this is not always easy to organise.
So the question is whether this can be made easier?
CGM can predict risk of progression from stage 1/2 to stage 3 type 1 diabetes
There were already data in 2019 that CGM can predict evolution from stage 1/2 to stage 3 (Steck et al. JCEM 2019)
and at the ATTD2023, 2 recent and larger studies were cited that further demonstrated this.
#1 In the Automimmunity Screening for Kids (ASK) Study, n=91 children with diabetes-related autoantibodies received a CGM for an average of 6 months.
- 18% progressed to stage 3 diabetes after an average of 4.5 months.
- Compared with the children who did not progress to stage 3, those who did progress to stage 3 had:
- a higher average glycaemia,
- a higher glycaemic variability,
- and a higher time >140 mg/dl or 7.8 mmol/l.
The study concluded:
- that a time >140 mg/dl or 7.8 mmol/l of >10% is associated with a higher risk of progression to stage 3 within the year in children with positive autoantibodies
- that CGM would be used in monitoring children at increased risk of stage 3 diabetes,
- and that CGM could be used as a possible inclusion criterion in prevention studies.
#2 In the CGM Metrics Indentify Dysglycaemic States in Participants From the TrialNet Pathway to Prevention Study, n=105 relatives of people with type 1 diabetes received a check of their diabetes-related antibodies, a CGM for 7 days and an OGTT every 6 months.
On this basis, n=53 people with stage 1 diabetes were identified, and n=42 people with stage 2 diabetes.

Analysis of the CGM data showed that you were at increased risk of evolution from stage 1 or 2 to stage 3 type 1 diabetes in the following cases:
- at a time ≥140 mg/dl or 7.8 mmol/l of ≥5%
- or a time ≥160 mg/dl or 8.9 mmol/l of ≥8%.
So both studies show that CGM can predict the risk of stage 3 diabetes in people with stage 1 and stage 2 type 1 diabetes.
The glycaemia from when exactly your risk is increased does differ between these studies.
CGM may also be able to predict stage 1 diabetes risk in first-degree relatives
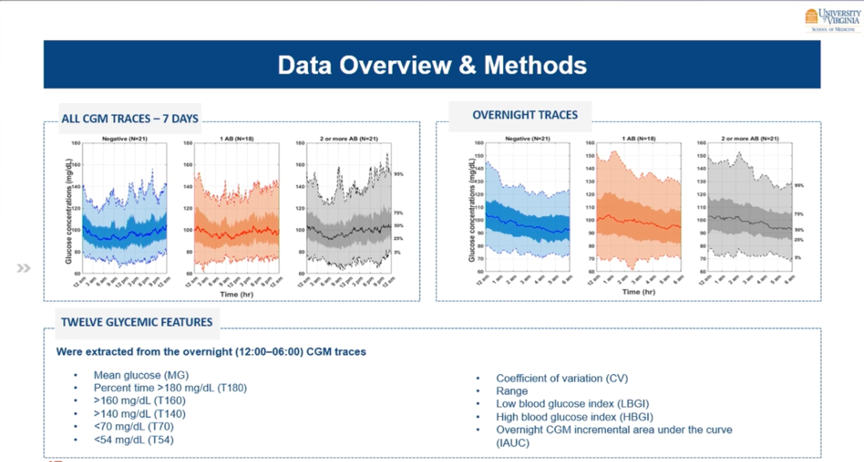
Also shown at ATTD2023 were results from a study from Virginia, which investigated whether you can use CGM to detect people at increased risk of type 1 diabetes.
For this, they gave 60 first-degree relatives of people with type 1 diabetes a CGM for 1 week.
- 1/3 had no autoantibodies (n=21),
- 1/3 had 1 autoantibody (n=18),
- and 1/3 had ≥2 autoantibodies (n=21).
At first glance, their CGM curves appear similar, but logarithmic regression models identified a parameter that showed an association with the presence of the autoantibodies. Indeed, the iAUC (nocturnal incremental area under the curve) was higher in those with autoantibodies.
So perhaps CGM could eventually also be an alternative for repeated monitoring of autoantibodies in the blood of first-degree relatives of people with type 1 diabetes. After all, autoantibody detection is not cheap and is not always reimbursed.
3. CGM to predict gestational diabetes
The number of people with gestational diabetes in America increased by 30% between 2016 and 2020 (Gregory et al. CDC 2022).
Screening for gestational diabetes is important because the condition can affect both maternal and infant health.
Early detection and treatment can reduce the risk of complications and promote a healthy pregnancy and delivery.
However, there is no global consensus yet on how best to screen for gestational diabetes now, although there are usually regional agreements.
Usually, screening for gestational diabetes is done at 24-28 weeks' gestation with an OGTT (Oral Glucose Tolerance Test).
In the GLAM (Glucose Levels Across Maternity) Study, n=760 women in Pennsylvania received a blinded Dexcom CGM at their first visit to the gynecologist in the context of pregnancy.
- The CGM was worn for an average of 68 days, mainly during the 2nd trimester.
- At 24-28 weeks, the women were given an OGTT that did or did not diagnose gestational diabetes.
- The study then looked at whether there was a difference in the sensor glycaemia of those who were diagnosed with gestational diabetes and those who were not.
People diagnosed with gestational diabetes had higher mean glycaemia on CGM
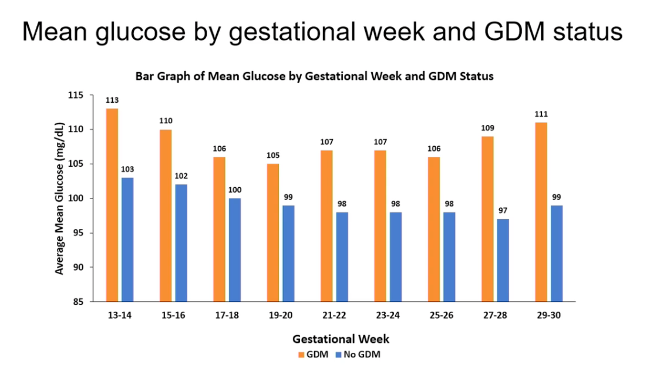
In the group that developed gestational diabetes, the average glucose between 13 and 30 weeks of pregnancy was higher than in the women who did not develop gestational diabetes.
- Around 13-14 weeks of pregnancy, this difference was 10 mg/dl or 0.5 mmol/l (113 mg/dl vs 103 mg/dl),
- around week 17-20 weeks' gestation, this difference was the smallest, at 6 mg/dl or 0.3 mmol/l,
- and around 27-30 weeks, the difference was the highest, at 12 mg/dl or 0.7 mmol/l.
People diagnosed with gestational diabetes had a higher time >120 mg/dl (6.7 mmol/l) on CGM
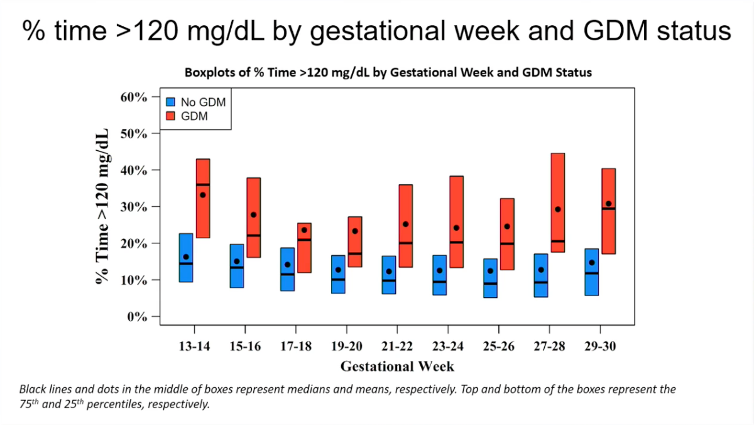
The time above 120 mg/dl or 6.7 mmol/l was also higher in the women who were later diagnosed with gestational diabetes, compared to those who were not diagnosed with gestational diabetes.
As with mean glycaemia, the smallest difference was seen at 17-20 weeks' gestation.
What does this mean?
It was natural to expect women who develop gestational diabetes to have signs of elevated glycaemia earlier in pregnancy.
However, it has so far not been shown that treating mildly elevated glycaemia in the first and second trimester has an impact on pregnancy-related complications.
We assume that we need to prevent especially the severe glycaemia dysregulation in the third trimester to avoid complications.
Further research will have to show whether detecting gestational diabetes earlier in pregnancy is useful, and if so, how to approach it.
Separately, it might make sense to explore whether screening with a CGM could replace standard screening with an OGTT.
After all, wearing a CGM is much easier and time-efficient than having to come to the hospital for a half-day OGTT.
Whether it will also be cost-effective is, of course, another question.
Perhaps one day women will have the choice of a copay for a sensor, or the standard screening with an OGTT?
First, of course, more data is needed.
4. CGM at SXSW2023
SXSW is a festival that brings together technology, music, film, culture and education in one event. It showcases local and international talent, innovation, creativity and diversity. It also provides opportunities for networking, learning, discovery and inspiration. SXSW is briefly considered one of the coolest festivals in the world. "You just have to be there" 😉
Nick Jonas is one of the celebrities using CGM and on SXSW2023 he made the case for its benefits.
The panel discussion with Nick Jonas, Dexcom COO Jake Leach and Dr Thomas Grace can be found here.
How nice to see the enthusiasm about CGM all the way to festivals like SXSW!
It is clear that the indications for CGM are getting wider and wider, and the momentum around CGM is ever-growing.
This quote from Dr Satish Garg (Editor in Chief of Diabetes technology and Therapeutics Journal) sums it up nicely:
"At the time of this writing, there were approximately more than 9 million people using a CGM, and this number is likely to increase in the next 5-10 years by two- or three-fold. Our predictions are that CGM will pretty much replace SMBG for insulin-requiring patients with diabetes. CGM will also become the standard for managing T2D, irrespective of the treatment regimen, and detecting prediabetes (both T1D and T2D) at an early stage so that necessary management can be implemented early. Is it time to call glucose a vital sign? It is likely that many people without diabetes (normal, healthy, non-diabetic individuals) may start using a CGM to look at their glucose profiles especially after meals. The future developments will hopefully see continuous ketone measurements (CKM) incorporated with a CGM to facilitate advanced diabetes management. As Time-in-Range (TIR) from CGM downloads is adopted more in clinical practice, it will likely replace the need for HbA1c measurements for day to day diabetes management." (Garg et al. DTT 2023)
Hopefully this hopeful message can inspire you :-)
Kind regards,





