DiabetesMine Innovation Days 2022 (english)
Dec 08, 2022
The 11th edition of the DiabetesMine Fall Innovation Days took place in San Francisco on November 17-18, bringing together entrepreneurs and patients with the MedTech industry.
DiabetesMine was founded by Amy Tenderich after her diagnosis with type 1 diabetes in 2003, and it's nothing short of inspiring to see how she organizes such fun events every year.
Some highlights of this year's event include:
- Biolinq measures glucose, ketones and lactate with microneedles
- Tidepool Loop nears FDA approval
- Glooko will further develop automatic advice
- BluHale = smart cap for Afrezza
- Art about diabetes technology
Read on below for more details!
1. Biolinq measures glucose, ketones and lactate with microneedles

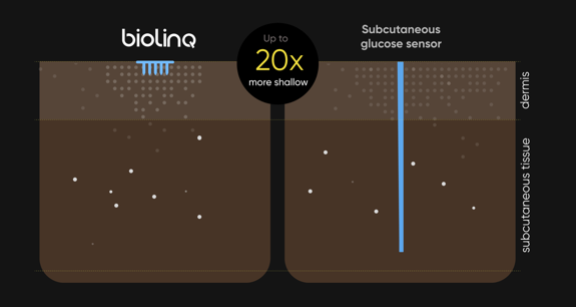
Biolinq has been working for 10 years on a micro-invasive glucose sensor that can measure not only glucose, but also ketones and lactate using microneedles.
There are not many results for the time being, apart from a small (n=15) study at the ADA 2020.
Despite this, they received 100 million dollars last year to further develop their product.
At the DiabetesMine Innovation Days they showed their renewed website, which is indeed very beautiful.
2. Tidepool Loop nears FDA approval
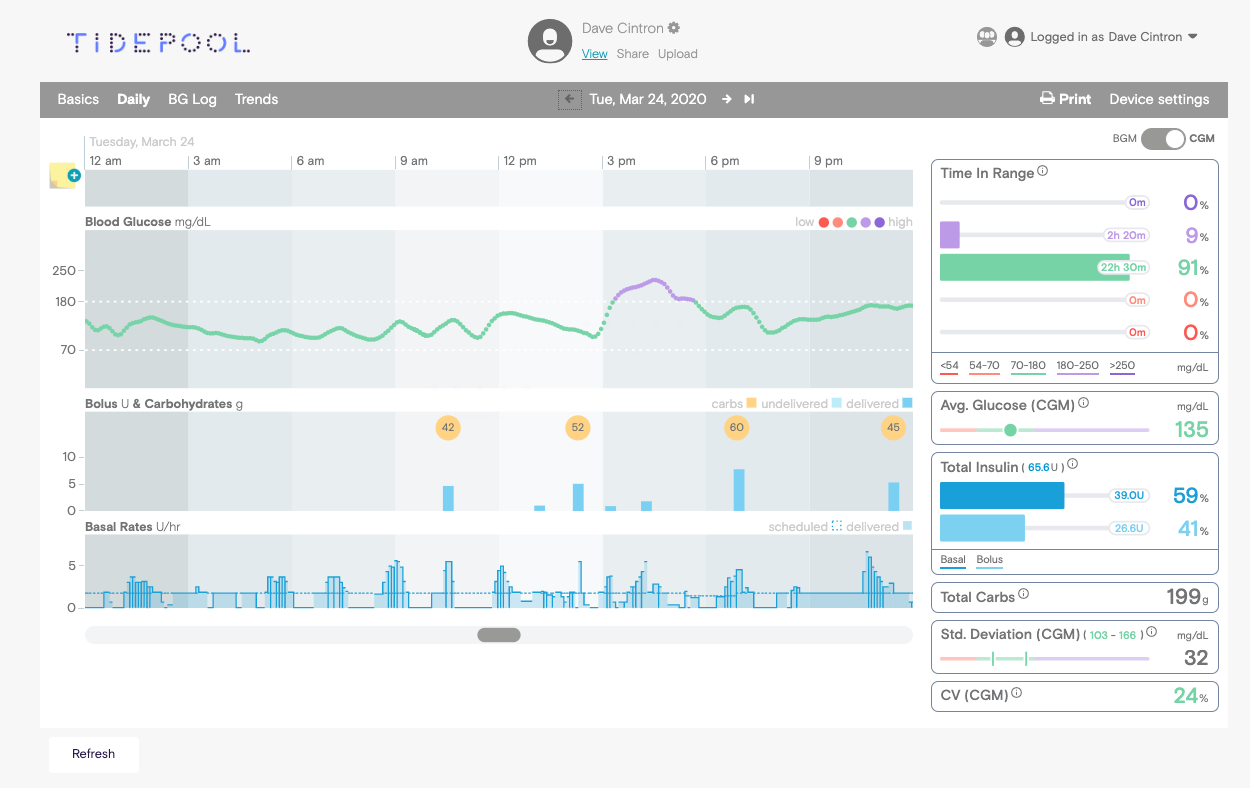
Tidepool is a readout platform for >75 devices (blood glucose meters, glycemic sensors and insulin pumps).
Just like Glooko.
At the moment their data platform is used by 336 000 people, of which 64% with DM1 and 28% with DM2 (and 8% unknown).
While Tidepool sees more and more users with DM2, with Glooko it would be the other way around (more shift to DM1).
Tidepool is a non-profit organization, and their reading platform is free.
However, they also have a paid version, which is Tidepool +.
With this premium version of Tidepool you get these extras:
- statistics at the population level (so that, as a healthcare provider, you have a quick overview of patients with severe hypoglycaemia, for example),
- built-in reminders to upload your device,
- single sign on,
- and priority troubleshooting.
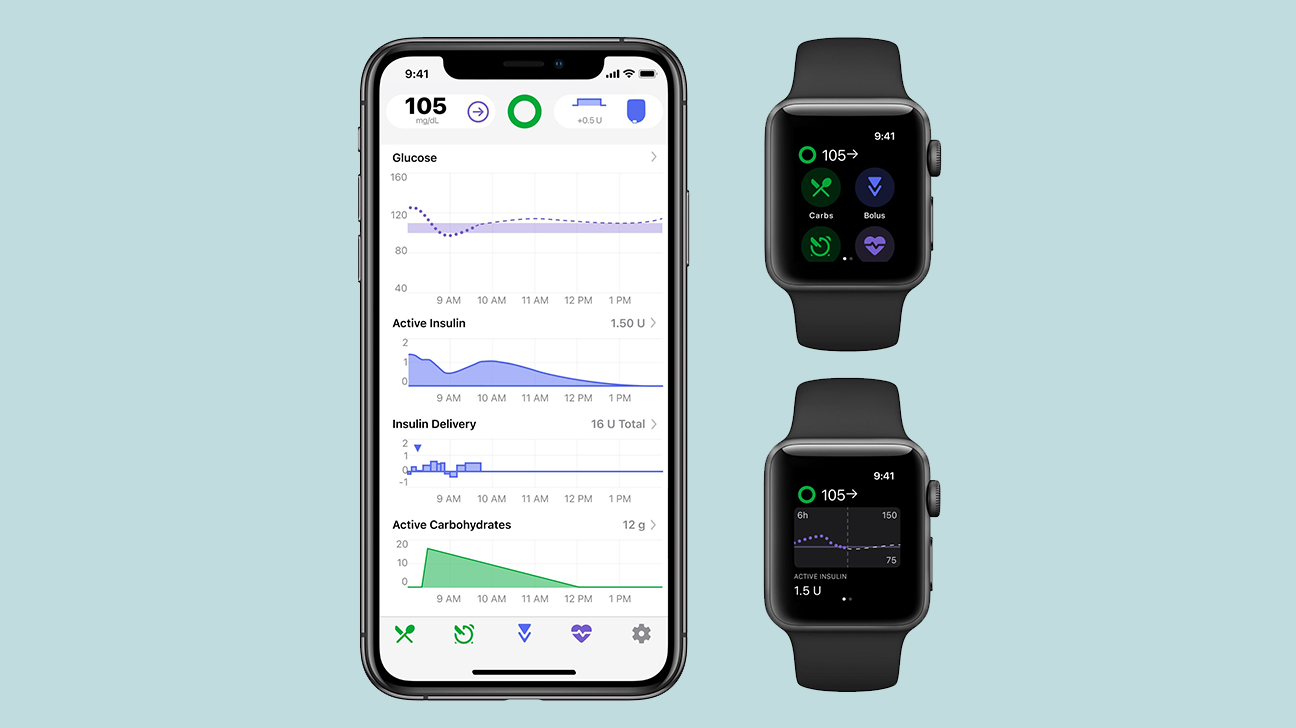
Most interesting is the fact that Tidepool wants to commercialize Loop's open-source AID algorithm as Tidepool Loop.
It filed an application for this with the FDA in 2020.
Reminder: Loop is an open-source AID system on an app on an iPhone.
- In terms of sensors, it can link to Dexcom sensors
- In terms of pumps, it can link to older Medtronic and Omnipod pumps, but there will also be a version for the connection to the Omnipod DASH.
At the DiabetesMine Innovation Days, Mr. Howard Look (CEO of Tidepool) said that the FDA label is expected any day now.
At that time, a Tidepool Loop app will be developed and will be available in the iOS App Store, so you no longer have to build it at your own risk.
(Tidepool Loop is said to be compatible with commercially available insulin pumps from Insulet and Medtronic, but it's not yet clear which exactly.)
3. Glooko develops automatic personalized advice
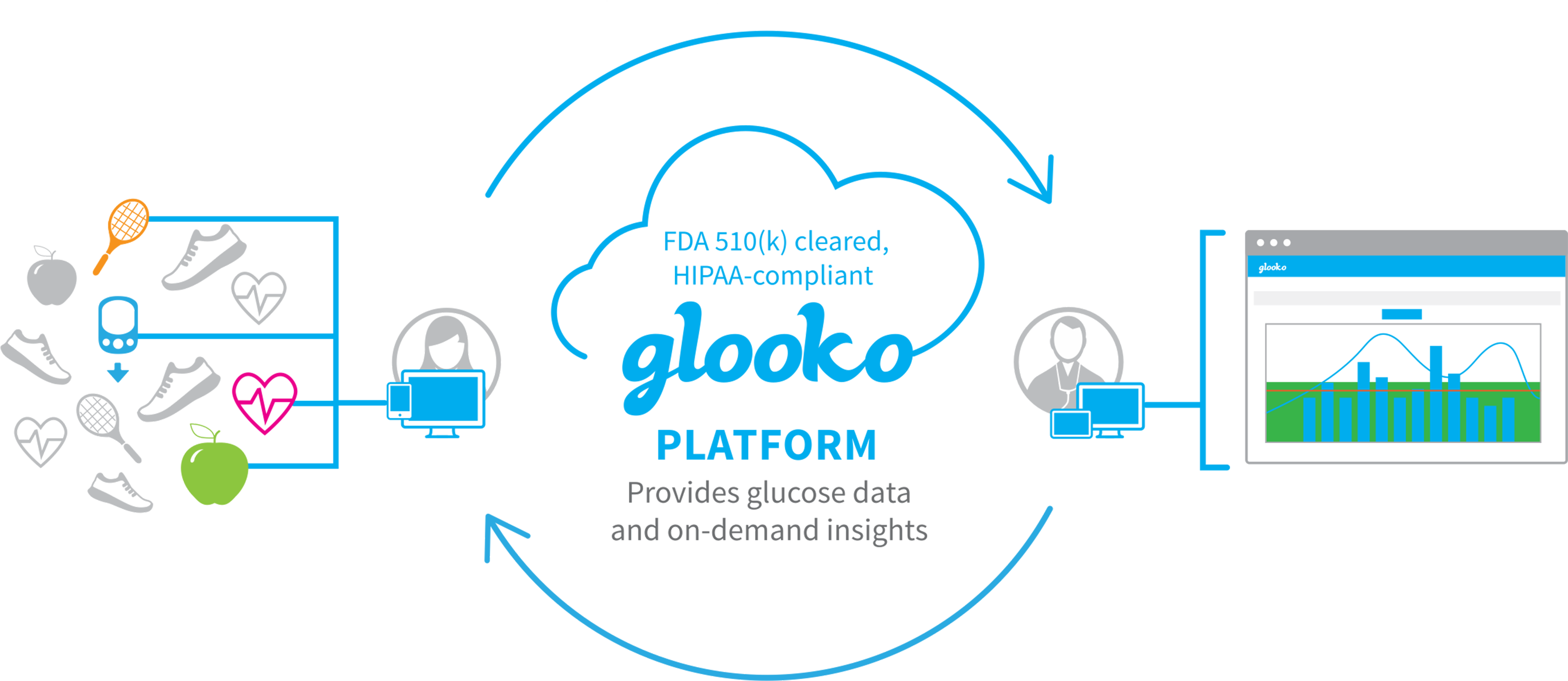
Glooko is a well-known readout platform for 184 devices (blood glucose meters, glycemic sensors and insulin pumps).
It is currently used by over 1 million people.

In January 2022 they bought xbird.
This is a German software company that can provide customized recommendations based on, for example, glycemic parameters ("AI enabled digital coaching").
The integration of these automatic advices will start from 2023, but for now only for research. The software does not yet have an FDA label.
It goes like this:
- Healthcare providers can target patients by device, type of treatment, type of diabetes, country/location, % that a device is worn, diabetes parameters (e.g. TIR, TBR), age, gender, language, etc..
- For a certain group they can choose that the patient receives a popup message on their Glooko app, a push notification on their phone, an email or a text message.
- This message can then be a text, a questionnaire, certain articles or videos with (for example) targeted education.
Of course, Glooko is not the only one developing personalized coaching.
For example, Medtronic launched "My Insights" in October 2022.
People in America who use the MiniMed 770G can already register for this.
They will then receive a monthly email with personalized advice based on their CareLink data.

Patients can also earn various fun badges as encouragement.
It looks like sensor data will certainly be interpreted partly automatically in the future, with
- on the one hand, personalized education/coaching (as in this example with Glooko and Medtronic)
- and on the other hand personalized advice on insulin dose.
4. BluHale = smart cap for Afrezza

Mannkind has developed a "smart device" that you can put on an Afrezza (inhalable insulin) inhaler.
This BluHale device will capture your inhaled insulin dose and send it to a BluHale app on your mobile phone via Bluetooth (for now with a delay of 3 hours).
- It also shows if you did the inhalation correctly.
- The device must be charged every 7 to 10 days.
The BluHale will be available in America from early 2023 (because Afrezza is only available there).
5. Art about diabetes technology
“Managing diabetes is not a science, it’s an art.” Weronika Burkot
Some artworks were also exhibited at the DiabetesMine Innovation Days.
Here are some gems about diabetes technology:
Pricks and Pokes (Dana Swann - @glucose101 - New York)

Tslim swim (Dana Swann - @glucose101 - New York)

Summer (Dana Swann - @glucose101 - New York)

We Are One (Weronika Burkot - blue_sugar_cube - Polen)

I hope you enjoyed these updates from the DiabetesMine Fall Innovation Days.
These events are critical in bringing entrepreneurs and patients together with the MedTech industry to create great diabetes technology.
And every event has its own emphasis, so you always encounter different things.
I personally thought the appropriate art exhibition was a very nice addition to the event.
Greetings,






