Key AID System Developments from ADA 2024
Jun 24, 2024
The American Diabetes Association (ADA) 84th Scientific Sessions is taking place from June 21-24, 2024, in Orlando, marking the largest global congress dedicated exclusively to diabetes.
This premier event features over 200 speakers and more than 2100 abstracts, offering a wealth of knowledge and advancements in diabetes care and research.

In this blog post, we will delve into the latest updates on Automated Insulin Delivery (AID) systems shared during the sessions.
Noteworthy highlights include:
- Medtronic showed the first user data of the MiniMed 780G with the new Simplera Sync sensor, showing an impressive Time in Range (TIR) of up to 80% in adults and 84% when using the recommended settings. They also presented a study of the Minimed 780G in individuals with type 2 diabetes achieving an average TIR of 80%.
- Insulet reported that training for Omnipod 5, whether in-person, virtual, or self-guided, yields similar outcomes. Promising results were observed for the Omnipod 5 in people with type 2 diabetes, including those on basal insulin only.
- Beta Bionics revealed that both in-person and virtual training, whether conducted by an endocrinologist-led team or in primary care, provide comparable results.
- Embecta shared why people with type 2 diabetes might benefit from a 300 U or higher patch pump, something that they aim to bring to the market.
Read the first report off the ADA congress now to get a glimpse into the future of diabetes management.

Medtronic

#1 MiniMed 780G with Simplera Sync sensor and EWIS leads to average TIR of 80%-84%
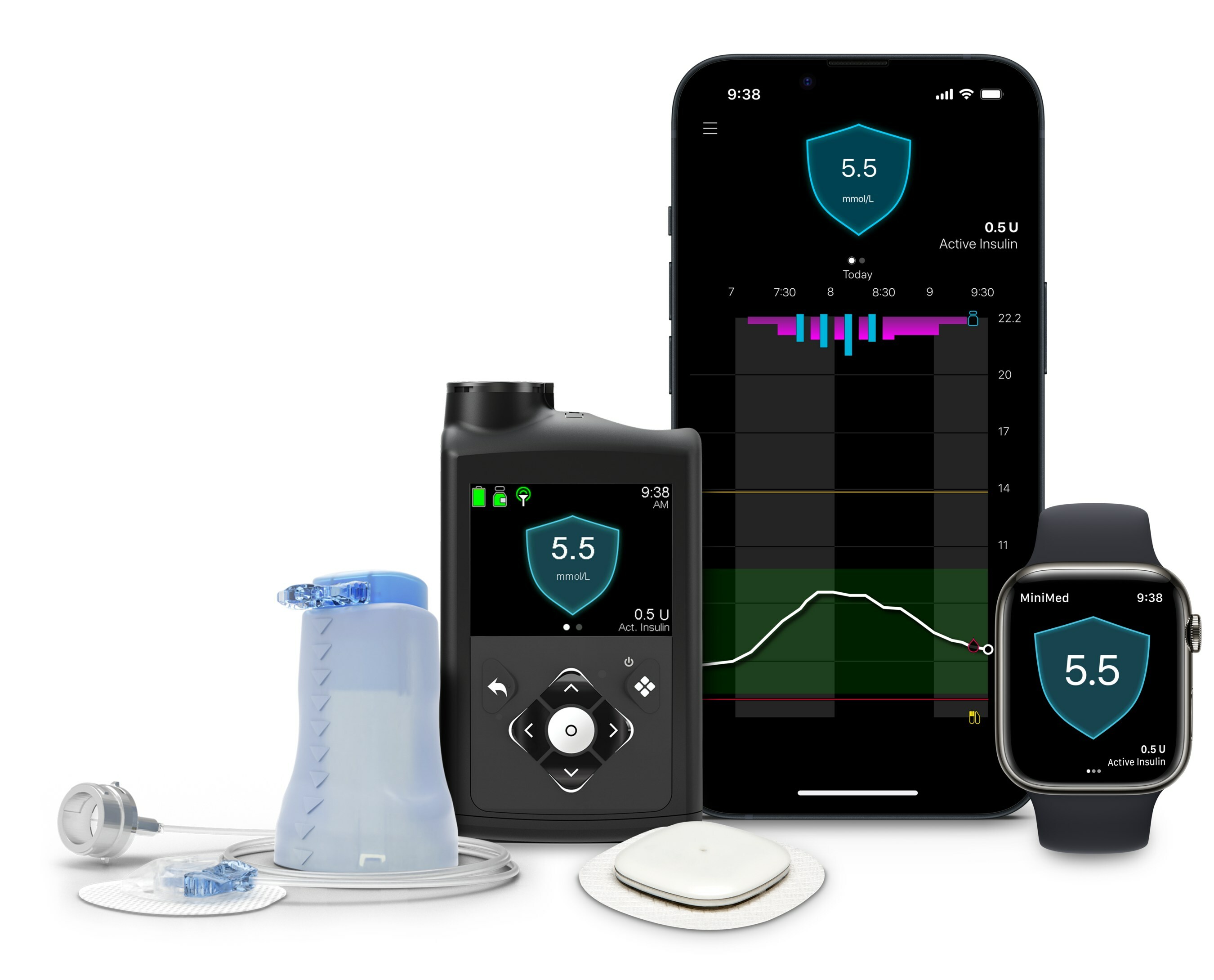
Medtronic unveiled the results of the MiniMed 780G system, featuring the new Simplera Sync sensor and a 7-day infusion set, from a prospective study.
The study involved 250 individuals with type 1 diabetes, aged 7 to 80 years, who used the system for three months.
The results (TIR = Time In Range, TBR = Time Below Range) are as follows:
- Youth (7-17 years) n=109: TIR increased from 54% to 71%, while TBR remained stable (1.6% to 1.9%).
- Adults (18-80 years) n=107: TIR improved from 67% to 80%, with no significant increase in hypoglycemia (TBR from 1.7% to 1.5%).
A subset of participants used the recommended settings (glucose target 100 mg/dl and active insulin time of 2 hours), showing even better results:
- Youth (7-17 years) n=41: TIR reached on average 75%, TBR 1.9%.
- Adults (18-80 years) n=45: TIR reached on average 84%, TBR 1.7%.

It's remarkable that the average TIR in this study was 80%, rising to 84% with the recommended settings!
In a recent real-world study, the MiniMed 780G demonstrated an average TIR of 72.3%.
We are optimistic that the updated MiniMed 780G system, with the new sensor, infusion set, and potential algorithm enhancements, will yield even better real-world outcomes.
#2 Prospective study of MiniMed 780G in type 2 diabetes shows TIR of 80%
Currently, Automated Insulin Delivery (AID) systems are approved only for individuals with type 1 diabetes,
but multiple studies have demonstrated their effectiveness in managing glucose levels for people with type 2 diabetes as well.
AID system manufacturers are now racing to collect the necessary data to submit their systems for approval to the FDA and European authorities for this new indication.
Medtronic, for example, is recruiting for a large study involving 575 participants with type 2 diabetes, which will be used to submit the MiniMed 780G for FDA approval for type 2 diabetes.
At ADA 2024, results from a smaller study involving 95 adults with type 2 diabetes were presented.
The study included:
- 58 people using multiple daily injections (MDI)
- 30 people using an insulin pump
- 7 people using an AID system
Most participants were also using other non-insulin glucose-lowering therapies.
After three months of using the MiniMed 780G system, the results showed:
- TIR increased from 72% to 80%, while TBR remained low (0.4% to 0.3%)
- HbA1c decreased from 7.9% to 7.2%
- Total daily insulin dose increased from an average of 77 units/day to 92 units/day
- Participants' weight did not increase significantly (average from 105 to 107 kg)
- Participants also reported lower diabetes distress and reduced fear of hypoglycemia at the end of the study period.

Given the increase in the total daily insulin dose, we are keen to see the 6- and 12-month results regarding participants' weight.
While it is normal for insulin needs to increase in order to achieve better glucose control,
it's important to avoid "overtreating" individuals with type 2 diabetes with insulin, as this could lead to a cycle of weight gain and increased insulin requirements.
Insulet

#1 In-person, virtual and self-guided training of Omnipod 5 give similar glucose results

Users new to the Omnipod 5 AID System can complete training and onboarding via personalized trainer-led education, virtually or in-person,
or for those upgrading from an earlier Omnipod System, online, self-guided training modules can be completed.
Insulet showed results of over 31,000 people using the Omnipod 5 AID System that TIR and TBR results were similar across all groups.

These results support the effectiveness of virtual and self-guided training for AID systems
and support expansion of these options to facilitate convenient and timely access to AID technology.
#2 Omnipod 5 for people with type 2 diabetes submitted to FDA based on data of the SECURE-T2D trial
Insulet is the first to complete a large trial of their AID system
and submit the Omnipod 5 to the FDA for approval for people with type 2 diabetes.
At ADA 2024, the results of the SECURE-T2D trial were presented:
305 adults with type 2 diabetes used the Omnipod 5 system for 3 months
- 21% were on basal-only insulin
- 73% were on basal-bolus insulin
- 6% were using an insulin pump before the study
Participants also used non-insulin glucose-lowering therapies, with 55% using GLP1-agonists and 44% using SGLT2-inhibitors
Participants could choose how to bolus:
- Some counted carbs and entered the grams of carbs they were going to eat
- Others used meal announcements with fixed doses or small/medium/large options
- Some only bolused for blood glucose corrections

Results after 13 weeks of using the Omnipod 5 system showed:
- TIR increased from 45% to 66%, while TBR remained low at 0.2%
- HbA1c dropped from 8.2% to 7.4%, with the most benefit seen in people with a higher HbA1c at baseline
- Unlike the results with the MiniMed 780G system, the average total daily insulin dose decreased from 0.8 U/kg/day to 0.57 U/kg/day, a discrepancy that remains unexplained
- No weight change results were disclosed

Using the Omnipod 5 resulted in significant and clinically meaningful improvements in diabetes distress,
with a notable reduction in the percentage of participants experiencing high diabetes distress (T2-DDAS total score ≥2.0).

In Insulet’s press release on June 21, 2024, Dr. Trang Ly, Insulet Senior Vice President and Medical Director, concluded:
“These data demonstrate that simple, easy-to-use AID technology, such as Omnipod 5, can be adopted by a broad population of people with type 2 diabetes and improve their lives.”
Beta Bionics

# Similar results with the iLet after in-person / virtual and endocrinology / primary care training

In the US, 50% of people with type 1 diabetes are being treated by primary care physicians.
Although AID systems are usually started in endocrinology clinics, the iLet is designed to be easy enough to do the onboarding directly via the primary care physician.
Beta Bionics did a small cross-over study in 40 adults with type 1 diabetes who got 14 days of usual care and 14 days of using the iLet, or the other way around.
- 10 people were trained by the endocrinology clinic in-person
- 10 people were trained by the endocrinology clinic virtually
- 10 people were trained by the primary care team in-person
- 10 people were trained by the primary care team virtually

The results were presented at ADA.
There were no differences in average blood glucose between the 4 groups,
and all groups achieved a significantly better TIR without increase in hypoglycemia.


These findings suggest that AID systems with a simple initiation process like the iLet system,
might expand AID access for people who only have access to primary care physicians.
Embecta
# Quantified data on total daily insulin need of people with T2D show a need for 300U insulin patch pumps
Embecta presented data from a retrospective observational study evaluating potential factors impacting total daily insulin dose (TDD).
The study analyzed over 41,000 adults with type 2 diabetes using multiple daily injections (MDI) treatment, sourced from the IQVIA ambulatory electronic medical record dataset (01/2017 - 07/2022).
Key findings included:
- The mean TDD in the US was 96 units per day.
- Distribution of insulin use:
- 23% used <50 units per day
- 41% used 50-100 units per day
- 21% used 100-150 units per day
- 15% used >150 units per day

Significant predictors of lower TDD were:
- Female sex (vs. males)
- African-American race (vs. white)
- US Census Regions other than the South (vs. South)
- Utilization of sulfonylureas or metformin in the 6-month pre-index period
- Utilization of 2-3 additional diabetes medications (regardless of class) in the pre-index period (vs. MDI only)
Predictors of greater TDD included:
- Age 30-64 years (vs. ≥65 years)
- Increased BMI (3% increase for each unit increase in BMI)
- Utilization of GLP1 or SGLT2 in the 6-month pre-index period
Based on these mean TDDs, Embecta estimated the number of people for whom 200-unit (u) and 300u insulin reservoirs would be sufficient for different wear times
and the number of patch pumps needed over time.

For 72-hour wear:
- 200u reservoirs were sufficient for 15,612 (38%) adults
- 300u reservoirs were sufficient for 26,290 (64%) adults
For 48-hour wear:
- 200u reservoirs were sufficient for 64% of adults
- 300u reservoirs were sufficient for 85% of adults
For 24-hour wear:
- 200u reservoirs were sufficient for 94% of adults
- 300u reservoirs were sufficient for 99% of adults
As evidence grows regarding the benefits of AID systems for people with type 2 diabetes,
it's important to note that only 38% of adults with type 2 diabetes in the US may find a 200-unit insulin patch pump sufficient for three days.
Therefore, developing insulin patch pumps with larger reservoirs, such as the one from Embecta, is a crucial step forward.
Conclusion

To conclude, the ADA 2024 conference delivered numerous new insights and promising trial results.
We are optimistic that the average TIR can reach as high as 80% to even 84% in individuals using the new MiniMed 780G system.
It is also encouraging to see significant steps being taken to extend this technology to people with type 2 diabetes.
These congresses serve as a powerful reminder that we are not alone.
Many dedicated individuals work tirelessly every day towards treating and curing diabetes, and many of us strive to help in any way we can.
"It takes all of us to end diabetes."
Step by step, we can do it.
To learn more about Automated Insulin Delivery (AID) systems and elevate your expertise in diabetes technology, click here to start your 7-day free trial of the Diabetes Technology Expert Program.
With over 20 hours of video programs and courses covering more than 25 glucose sensors, insulin pumps, and AID systems, this is the most comprehensive platform available to skill up quickly and effectively.
Join now and become a true expert in diabetes technology!
Kind regards,





