Medtronic's ADA 2023 Investor Day
Jul 27, 2023
Welcome to the update on Medtronic's ADA 2023 Investor Day held on June 25, 2023.
During this event, Medtronic showcased its latest innovations and developments in the diabetes management space,
- unveiling the new 800-series pump
- and highlighting their MiniMed 780G algorithm.
- Additionally, the company shared insights into their projections for the adoption of connected devices and remote monitoring technology in diabetes care.
Here you can find the slides and webcast.
1. New 800-series Pump
"We have a number of pumps coming forward. Part of the reason why we're making some changes here, is the feedback from folks with type 1. They want to keep things as discete as possible with their diabetes and take more time living their own life. As a result of that, what you can see on both of these pumps, they no longer have screens on them." - Ali Daniaty, SVP, Product Innovation & Operations, Diabetes at Medtronic's ADA 2023 Investor Day June 25, 2023
During Medtronic's ADA 2023 Investor Day on June 25, 2023, the company unveiled its latest innovation: the 800-series pump.
This new tubed pump, sets itself apart by operating without a screen.
Medtronic's presentation didn't include any pictures of handsets, indicating their focus on investing in full phone control and developing an accompanying app.
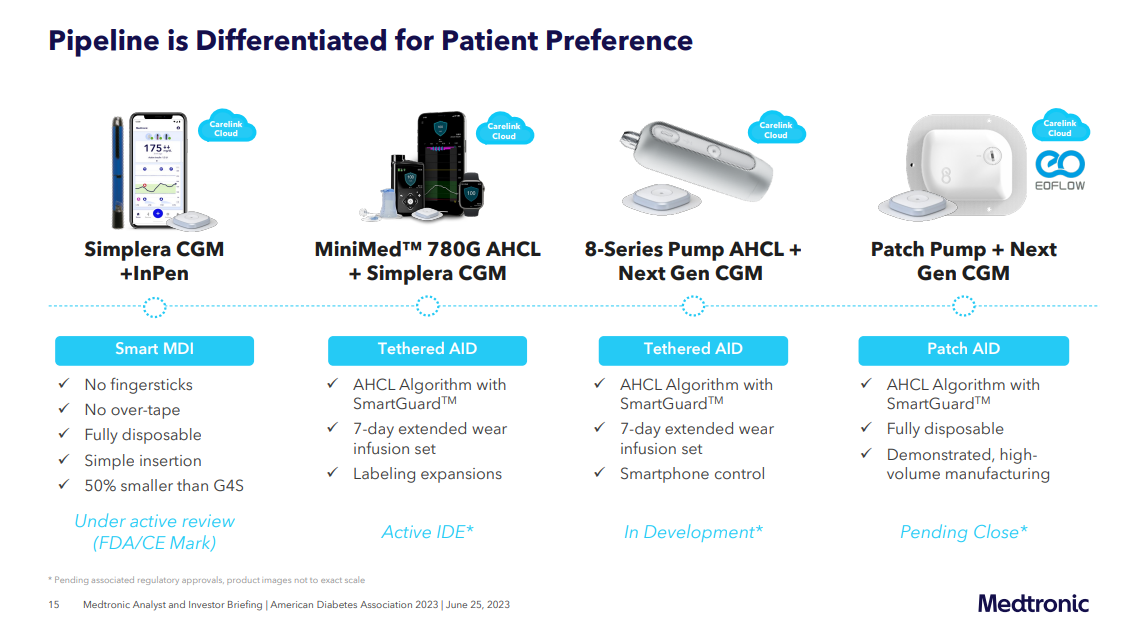
Compared to the currently used MiniMed pumps in the 600- and 700-series, the 800-series pump is impressively compact, being only half their size.
- It incorporates Medtronic's patented AHCL technology and integrates their advanced CGM known as "Instinct."
- The pump features a single light, which can display different colors such as green, orange, or red.
- Additionally, it will be equipped with a rechargeable battery that can reach full charge within 20 minutes.
- The new pump will be compatible with the current extended wear infusion sets and insulin reservoirs, so users will still have to manually fill their insulin reservoirs.
Medtronic also showed its acquisition of the EoPatch insulin pump in their new pipeline, although the acquisition is not closed yet.
- The new EoPatch pump which will be integrated with Medtronic's AID algorithm and next-gen CGM.
- Medtronic has no intention of changing the form factor of EOPatch, because EOFlow’s high-caliber manufacturing was one of the reasons Medtronic was initially interested in the company.
As for the next-gen CGM, known as "Simplera" when used independently and "Instinct" when paired with an insulin pump, Medtronic shared some progress.
- Submissions to the FDA and European regulatory authorities for the standalone CGM have been made, and the company is eagerly awaiting approval.
- Concerning the submission for the CGM paired with the Minimed insulin pump, the pivotal study is ongoing. The trial for adults was fully enrolled within a month, and enrollment for the pediatric cohort has commenced.
Despite the comprehensive presentation, specific launch dates for the new pumps and next-gen CGM were not disclosed during the event.
2. Medtronic positions their MiniMed 780G as the World's Most Responsive Algorithm
"The 780G looks the same on the outside, but it is completely different and revolutionary on the inside. And the data that we're presenting in terms of outcomes as well as burden simplification is really unmatched. You've seen the succes that we've had in Europe in prior earnings discussions and the early indication that we're seeing in the US is very positive, exceeding our own internal expectations. [...] The reason why the outcomes are so good is because of our unique meal detection algorithm." - Que Dallara, EVP & President Diabetes at Medtronic's ADA 2023 Investor Day June 25, 2023
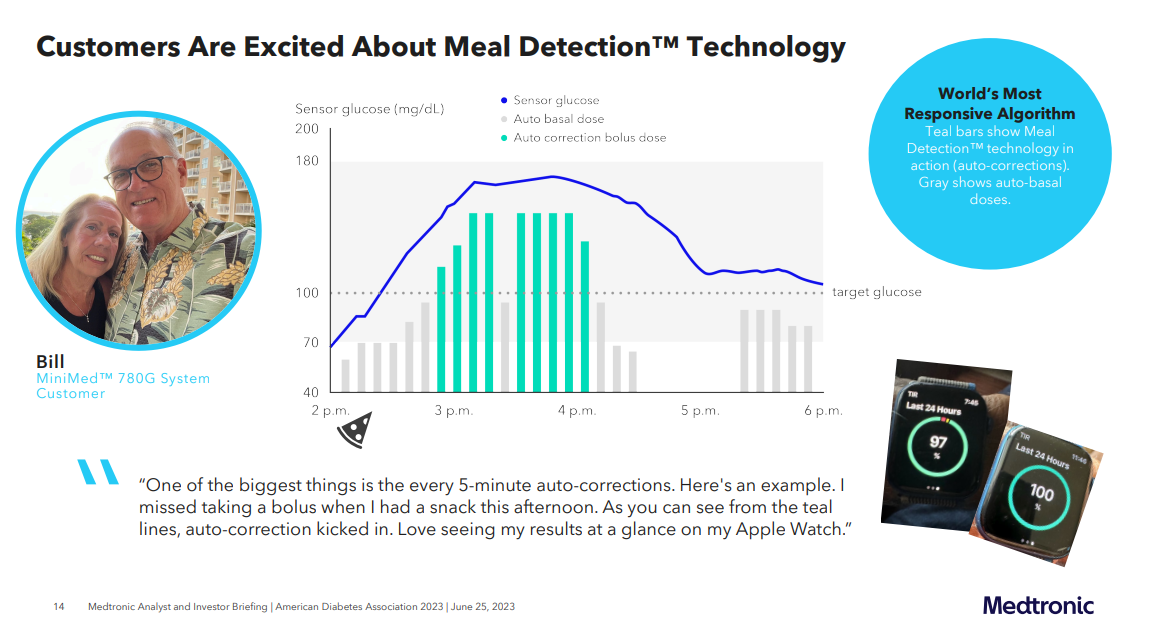
With the recent approval of the MiniMed 780G in the US, Medtronic is actively promoting the system, positioning themselves as having the best algorithm available.
This sentiment is shared by many physicians who work with the system.
However, determining whether this claim is true or not presents challenges.
Real-world studies have shown that Medtronic indeed demonstrates impressive Glycemic Management Indicator (GMI) and Time in Range (TIR) results, as follows:
- For patients aged 15 years or older: GMI 6.8%, TIR 76.5%, TBR 2.3%
- For patients under 15 years old: GMI 6.8%, TIR 73.9%, TBR 3.2%
Nonetheless, the accuracy of glycemic results heavily relies on various factors such as the patient population, baseline HbA1c levels, age, and user interactions with the system.
Since studies are conducted on different populations, making comparisons and definitive conclusions are theoretically not possible.
Furthermore, some users have noticed discrepancies between sensors,
- reporting that FreeStyle Libre sensors tend to show slightly lower glycemic values compared to self-monitoring blood glucose (SMBG),
- while Dexcom sensors do not exhibit the same issue.
- There are also some critic's speculations that Guardian sensors used with the MiniMed 780G might measure on the low side, potentially influencing the high TIR results seen with this system.
However, these remain as speculations until real comparison studies are conducted.
In conclusion, it's still too soon to know which closed-loop algorithm gives "the best" glycemic results.
3. Medtronic estimates 55% of people with diabetes on intensive insulin to be on "smart dosing systems" in 2030
"Remote monitoring is going to become a bigger deal. It's better now. [...] We have too much data in the world. We need insights that are actionable. I think with what we do [...] with AI and cloud computing, it's an exciting time overall for medtech monitoring. It's going to take some time for this to play out, but you're seeing leading indicators for this." - Geoff Martha, Chairman & CEO Medtronic at Medtronic's ADA 2023 Investor Day June 25, 2023
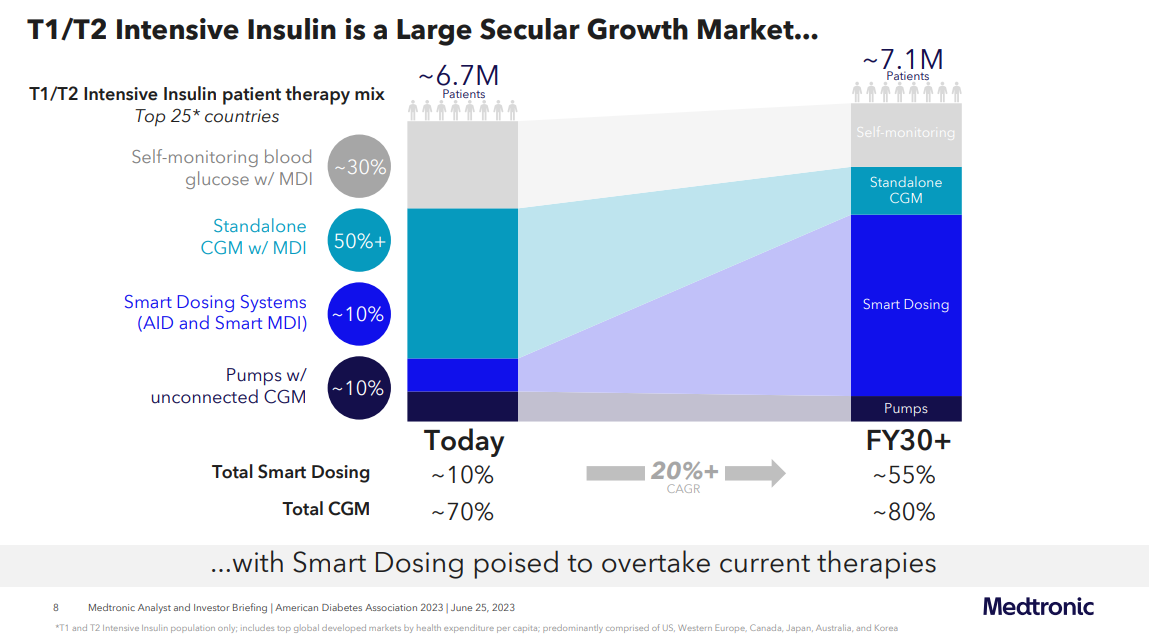
Regarding the connected pump and connected insulin pen market, Medtronic anticipates a remarkable increase in technology penetration in people using intensive insulin.
- It foresees the adoption of "smart dosing systems" to rise from roughly 10% to approximately 55% by 2030 in all people with type 1 and type 2 diabetes on intensive insulin - across the top 25 countries.
- Similarly, Medtronic projects an expansion in CGM penetration from about 70% to around 80% over the next seven years and beyond.
In the competitive landscape, Medtronic has demonstrated its strength, capturing about 40% of new pump starts in the top six Western European countries in the second half of 2022.

Mr. Geoff Martha, the CEO of Medtronic, emphasized Medtronic's unwavering commitment to its diabetes business, doubling down on investments in assembling a comprehensive ecosystem of durable pumps, smart pens, patch pumps, sensors, and algorithms.
With multiple programs under development, the company is taking an offensive approach in this field.
Medtronic's ADA 2023 Investor Day brought to light the company's commitment to continuous research and development for diabetes management solutions.
The introduction of the new 800-series pump, the impressive performance of the MiniMed 780G algorithm, and their projections for increasing adoption of smart dosing systems and remote monitoring technology reflect Medtronic's dedication to empowering individuals living with diabetes.
As they strive to create innovative products and embrace cutting-edge technologies, Medtronic's unwavering focus on improving diabetes care is evident, promising a brighter future for patients worldwide.
If you don't want to miss out on any updates in the exciting field of diabetes technology, make sure to click HERE to subscribe to our blog and stay informed.
Kind regards,