The future of diabetes: technology update
Oct 14, 2022If you're a health care provider or patient with diabetes, then you know that staying on top of the latest technology is critical to managing the condition.
Here's 3 updates on promising new technologies for diabetes management:
- Dexcom G7 Launches in UK, Ireland, Germany, Austria and Hong Kong
- Two new international consensus reports on AID systems
- No difference in time in range between open-source and commercial AID systems
If you want to know when the Dexcom G7 is coming to us, where you can find the latest guidelines on AID systems and whether open-source AID systems are really better than the commercial AID systems, read on!
1. Dexcom G7 Launches in the UK, Ireland, Germany, Austria and Hong Kong
As a reminder, the main advantages of the Dexcom G7 over the Dexcom G6 are:
- that it is 60% smaller than the Dexcom G6
- and that it only has a warm-up time of 30 minutes instead of 2 hours.
When will the Dexcom G7 be available?
9 months after receiving the CE label, Dexcom G7 will finally be launched.
Launch was announced on 10/4/2022 in UK, Ireland, Germany, Austria and Hong Kong.
New Zealand and South Africa will follow in the coming weeks, with other countries "soon" to follow.
I suspect that the Netherlands (and Belgium) will have to wait until 2023.
However, the fact that the Dexcom G7 will now be sold at the same price as the Dexcom G6 is not good news for Belgium. In Belgium, the Dexcom G6 is only available in combination with the Tandem Control IQ and Diabeloop, only for about 1200 patients in the "GDT centers".
However, at this time there is no integration of the Dexcom G7 into Automated Insulin Delivery (AID) systems.
No integration of Dexcom G7 in AID systems yet
At the moment there are only plans for integration of the Dexcom G7 in 2 AID systems:
- Tandem Control IQ
- and Omnipod 5.
It is still unclear whether Diabeloop and CamAPS FX (or mylife Loop) will ever work with the Dexcom G7.
Dexcom G7 will only be able to be integrated into these 2 AID systems 6 months after FDA approval.
However, the FDA label was denied this summer, and Dexcom said they would modify the Dexcom G7's software and then resubmit the Dexom G7 to the FDA.
So this can take several months.
What can we expect in the future at Dexcom?
Dexcom is actively working on 2 additional functionalities for the Dexcom G7:
-
a direct-to-watch option, so you no longer have to carry your mobile phone to see your glucose data on your smartwatch. At the moment this is only under development for the Apple watch.
-
a sensor life of 15 days. Funny that this is just 1 day more than the 14 days of the other CGMs.
2. Two new international consensus reports on AID systems

Last month, 2 international consensus papers were published on AID systems:
- the "Consensus Recommendations for the Use of AID Technologies in Clinical Practice" was published 9/6/2022 in Endocrine Reviews.
- and "Automated insulin delivery: benefits, challenges and recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the EASD and the ADA." was published simultaneously in Diabetes Care and Diabetologia on 10/6/2022.
#1 Consensus Recommendations for the Use of AID Technologies in Clinical Practice
This consensus was formed from the discussions held at the ATTD of 2021.
75 endocrinologists participated, who were divided into 9 groups. Each group was assigned a separate topic:
- Evaluation of AID systems
- Summary of clinical evidence
- Target population for AID therapy
- Initial Aid system use
- Education, training and support
- Clinical Recommendations for AID use
- How to report and present AID data
- Psychological issues and PwD perspectives
- The future of AID: what will it look like?
The purpose of this article is to
- provide clinical guidelines for healthcare providers who want to work with AID systems,
- and to list the current evidence on AID systems for healthcare institutions considering who should be eligible for reimbursement of AID systems.
Unfortunately, the layout of the article is not yet finished, making the article not so easy to read. If you don't want to wait for a better layout, click here to read the article.
#2 Automated insulin delivery: benefits, challenges and recommendations
This article is an independent consensus of the "Joint Diabetes Technology Working Group" of the EASD and the ADA. Three researchers from both the EASD and the ADA were nominated to sit in this working group.
This is the 4th statement of this working group, the previous ones were:
- Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting and research needs (2015)
- Improving the clinical value and utility of CGM systems: issues and recommendations (2017)
- Diabetes digital app technology: benefits, challenges and recommendations.(2020)
The focus of the current article is on discussing the safety of AID systems.
The article is divided into the following chapters:
- Using time in range to contextualize the impact of diabetes technology
- Benefits of AID systems
- Limitations of AID systems
- Education and expectations
- Patient perspective
- Provider perspective
- Special populations: what is needed?
- Considerations for patient selection for current AID systems
- Safety aspects to be considered for AID systems
- Cu-ybersecurity, data privacy, data protection, GDPR and data donation
- Evaluation and approval of AID systems in the US and Europe
- Access to AID systems
- Liability
- DIY AID systems
- Consensus report recommendations
Curious about their decisions? => here you can read the article for free.
3. No difference in time in range between open-source and commercial AID systems
People on open-source AID systems often have a better time in range (TIR) than people who use a commercial AID system.
So you might wonder whether an open-source AID system might be better?
The CREATE trial already showed that open-source AID systems are just as effective as commercial AID systems
The CREATE (Community deRivEd Automated insulin delivery) trial was discussed at the ADA and EASD 2022, and published last month in the New England Journal of Medicine. An editorial about it appeared in the JAMA on 10/11/2022.

In this prospective study, 97 people with type 1 diabetes (7-70 years) were randomized to:
- AndroidAPS on a cheap cell phone, a Dana-i insulin pump and a Dexcom G6 CGM
- or "sensor-augmented pump therapy" (control group).
After 24 weeks, the TIR had increased from 61% to 71%.
At EASD 2022 it was reported that the TIR was similar after 48 weeks.
=> This TIR increase of approximately 10% is similar to the results we see with the commercial AID systems.
CODIAC study
The results of the CODIAC study (Comparison of 2 Different Hybrid Closed Loop Systems – AndroidAPS and Control-IQ in Patients with Type 1 Diabetes) were first shown at EASD 2022.
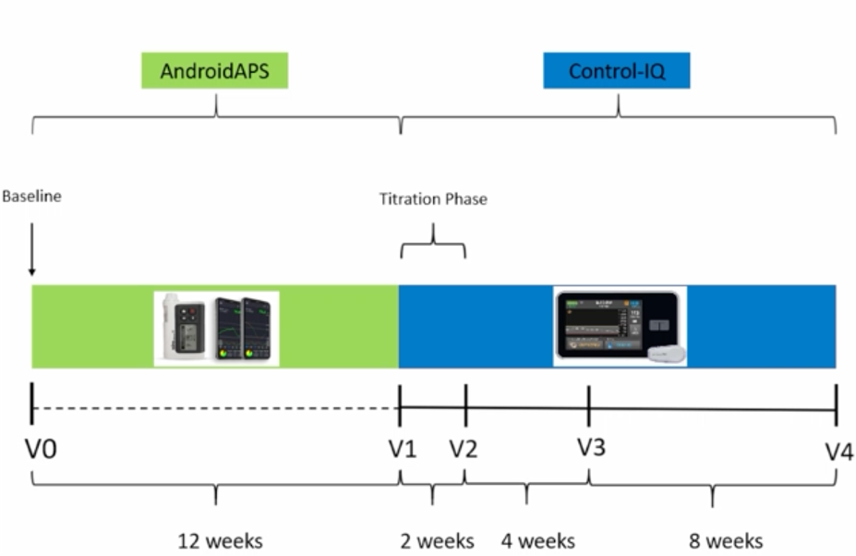
In this prospective study, 23 adults with type 1 diabetes who were taking AndroidAPS were switched to Tandem Control IQ.
The TIR on Tandem Control IQ was then compared with the TIR of the period when they were still using AndroidAPS.
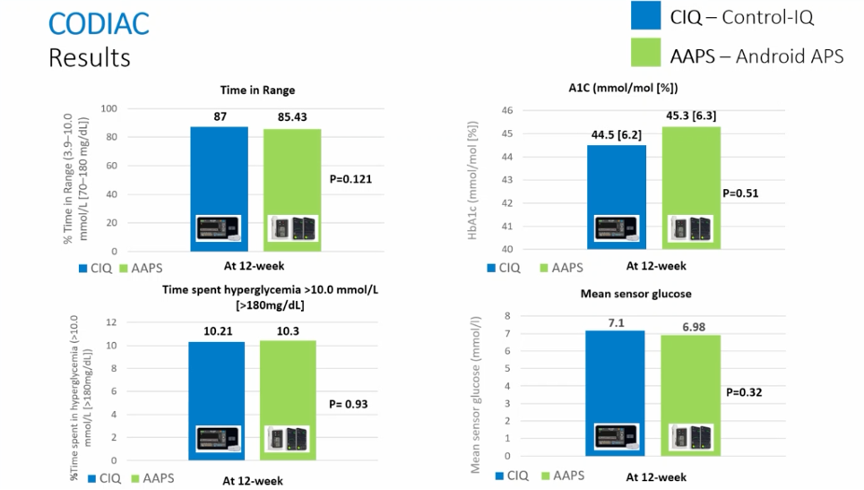
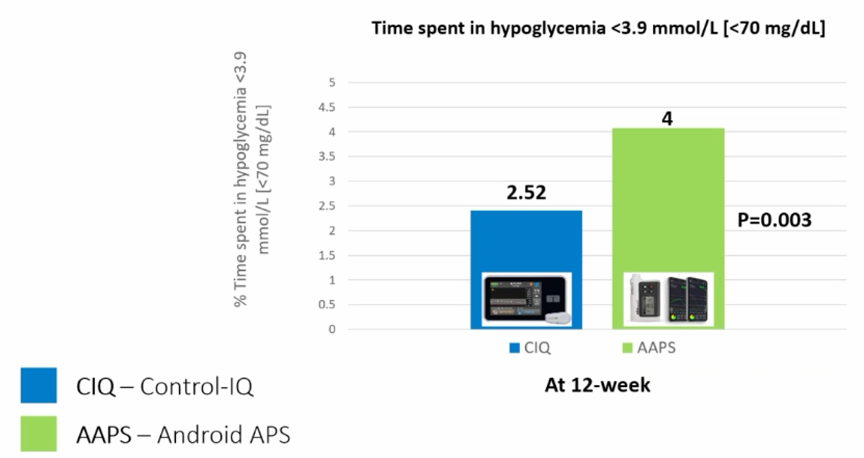
Results:
- The TIR on Control IQ was not different from that on AndroidAPS (85% and 87%), respectively.
- The time below range (<70 mg/dl, TBR) was lower on Tandem Control IQ.
Conclusion: open-source AID systems do not give better results than commercial AID systems
The CODIAC study is the first prospective study to compare an open-source AID system with a commercial AID system, and it shows that the algorithm of open-source AID systems is no better than the commercial AID systems, but no worse either.
(This small study group was, of course, exceptionally well controlled with a TIR around 85%, and it is not clear whether we can extend these results to other populations.)
Probably the better results we see in people on open-source AID systems are due to the kind of people who choose an open-source AID system. Often these are very motivated people who really want an extremely good glycemic control. They often opt for an open-source AID system to have even more control on their glycemia than is possible with a commercial AID system. They are well educated and understand very well the effect diet and exercise have on their glycemia.
They may achieve better results with their open-source AID system because they interfer more, and more correctly with their system: more boluses, more correctly estimate carbohydrates and more anticipation of glycemic fluctuations during exercise/illness. It has also been shown with commercial AID systems that the TIR is better the more boluses you give.
So it looks like the Dexcom G7 will finally be launched, even though it was denied the FDA label this summer. More and more articles are available that bundle the current evidence on AID systems and give us the necessary tools to implement them in a scientifically correct way. And finally, open-source AID systems appear to work just as well as commercial AID systems if you have them used by the same "type" of patients.
The result of an AID system therefore depends not only on the AID system itself, but also on the patient who will use it.
The more motivated you are, and the more you interact (adjust boluses/target) with the system, the better the results.
While it's important to include all of this in the education, you also need to maintain a balance between good glycemic control and your quality of life.
Not everyone will push their glycemic control to the forefront, or surely there may be times in your life when perfect glycemic control isn't the priority.
The clinical importance of a TIR >80% compared to a TIR of >70% is also not fully understood at this time.
In addition, open-source AID systems are still a viable option for some patients.
Although they may not give a better TIR result, they do provide more opportunities to interact with the system and, for example, to choose your own target value.
And they also give more choice in the pump and CGM that you might want to use.
So whether you choose an open-source or commercial AID system, this is still the "best" way to get your glucose under control if you have type 1 diabetes.
Kind regards,





