Update November 2021
Nov 29, 2021
I had hoped to be able to give you an update on diabetes technology every week, but corona throws a spanner in the works here 🤷♀️.
From quarantine I would like to give you some news about:
⭐Medtronic
⭐Tandem Control IQ
⭐Abbott
⭐Dexcom
⭐Miscellaneous
Just in time to end November, the one and only ✨diabetes month✨. 👊
Medtronic
![]()
🔹 At the ISPAD conference we saw real-world data from the MiniMed 780G in children under 15 years old (n=3211): their TIR increased from 62% to 74%. Although the results with closed-loop systems have always been worse in children than in adults, we now see for the first time that the results in children practically match those of adults (cfr results >EASD: TIR 76%). It was suggested to set the target value for the SmartGuard in children at 110 mg/dl, because this results in fewer hypo's in children (average 3.6% TBR at 100 mg/dl and 2.7% TBR at 110 mg/dl).
🔹The approval of the MiniMed 780G and Guardian 4 in the US has again been postponed to “the first half of 2022”. Only then (by April 2022) will Medtronic's new sensor be submitted to the FDA. The latter is no longer called “Synergy”, but rather “Simplera”.
🔹A study discussed at the Virtual Diabetes Technology Meeting (VDT Meeting) showed that use of the InPen (n=1681) was associated with 13% less severe hypos. Based on this, cost savings of $2176 per user per year were calculated. Hopefully this study can help to provide reimbursement in Belgium one day.🙏
🔹Medtronic is not standing still anyway, cfr they bought the intellectual rights of an implanted pump in September of this year, and that of a patch pump in October.
Tandem Control IQ
🔹CLIO study: new subanalysis of this real-world "Control IQ Technology Observational" study appear at every congress:
- ATTD: adults (n=700) have a mean TIR of 74%
- ADA: improvement across different racial and ethnic groups (n=1094)
- Keystone 2021: improving quality of life (DIDP scale from 4.79 to 3.26)
- ISPAD: children (6-17 years, n=365) have a mean TIR of 65%
- VDT Meeting: the rates of self-reported ketoacidosis, severe hypoglycemia and hospitalization in adults (n=1804) decreased significantly, independent of baseline HbA1c and prior pump use.
Closed-loop systems are therefore certainly not only useful for people with an HbA1c <8.5% or for people who already use a pump, which was sometimes thought in the past.
🔹In a prospective study in children 7-16 years (n=46), the TIR increased from 64% to 76% (also after 6 months), which is considerably better than the results in children in the CLIO study, possibly due to the more intensive training in a “virtual education camp”. So perhaps it makes sense to make education for closed-loop systems a bit more structured and standardized than is currently the case.
Abbott

🔹Libre3 is currently available in Germany, but it is still unclear when it will come to Belgium. Several people were able to test it out and were satisfied (eg Tom and Kamil). There is a report of a less good reliability the first day, and that the app is still only available for iOS. This is important because a reader is no longer available with Libre3.
🔹Abbott shows interest in ketone monitoring. The Libresensor can apparently be transformed into a continuous ketone meter (CKM) instead of a continuous glucose meter (CGM). A small study (n=12) with a modified Libresensor showed that the MARD was 14.4% for ketones >1.5 mmol/l. This is the first studied continuous ketone monitor in humans. More news about the usefulness of measuring biomarkers other than glucose in closed-loop systems will follow in one of the next blogs.
🔹A surprising study at the VDT Meeting showed that used Libre sensors can also be transformed into temperature sensors for insulin. After use, the Libresensor can be disinfected and the sensor removed with a ballpoint pen. With an Android smartphone you can reprogram the sensor via NFC via an open-source app. The reprogrammed sensor can then be used as a temperature sensor to monitor your insulin for up to 1 year.
🔹Health2Sync launches integration of the Libre CGM data in its app. Health2Sync is the leading diabetes management app in Japan, Taiwan, Hong Kong, Singapore and Malaysia, currently with more than 760,000 users. Since Health2Synch also has partnerships with Novo Nordisk, Sanofi, Fitbit, Ascencia and Cigna, users will be able to see a very nice integration of all their data.
Dexcom

🔹The Dexcom G7 will be launched at the end of this year in a number of countries in Europe, hopefully also in Belgium😍. As a reminder, the Dexcom G7 has a MARD of just 8.7%, is 60% smaller than the Dexcom G6, and has a warm-up time of just 30 minutes. The Dexcom G7 app provides a simple boot module, and displays TIR, GMI, and the AGP profile in the app, where it was previously only visible in the Clarity app. While ramping up production of the Dexcom G7, Dexcom is also working to approve Dexcom for people in the hospital and pregnant women.
🔹Meanwhile, Dexcom ONE has been launched in Bulgaria, Estonia, Latvia and Lithuania. This CGM, like the Dexcom G6, does not require calibrations, but the software is slightly simpler. The Dexcom ONE has no predictive alarms (but does have optional alarms), will not be compatible with closed-loop systems and offers no possibility for data sharing or remote monitoring. As with the Dexcom G7, the TIR and GMI are also visible in the Dexcom ONE app.
🔹This summer the FDA approved 2 software solutions for Dexcom:
-Real-time API was approved in July, allowing other apps to integrate Dexcom's real-time glycemic data. Previously, this was only possible with a delay of 3 hours. There are already 2 apps that successfully integrated the Dexcom data in this way: Garmin and Welldoc BlueStar
-In August, the app-in-app module was approved for people with type 2 diabetes who are treated "non-intensively". Normally, if you want to use the Dexcom data in another app, you always have to install both apps. With the app-in-app module you only need to install 1 app. The first app to take advantage of this is United Healthcare's Level2 program, an app/program in collaboration with Dr Jason Fung to reverse type 2 diabetes. Through their partnership with Livongo, Welldoc and Onduo, Dexcom similarly aims to help reverse type 2 diabetes.
Miscellaneous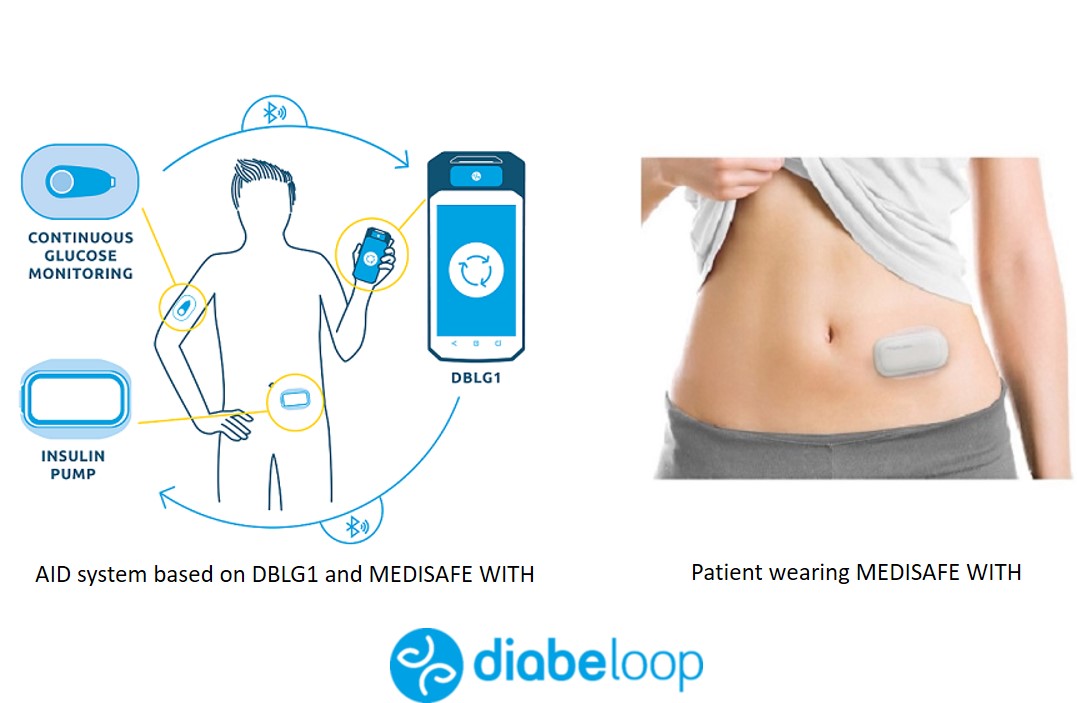
🔹Diabeloop-Terumo is coming to Europe. Diabeloop has been collaborating for some time with Terumo, a producer of an insulin patch pump (Medisafe With). The combination of Diabeloop with this "Medisafe With" insulin pump will probably become the 2nd closed-loop system with a patch pump (after Omnipod 5 that is expected next year in the US).
🔹GlucoMen Day CGM (Waveforms's Cascade CGM) mentioned at the VDT Meeting that they expect CE approval next month to use their CGM with only 1 calibration every 2 days instead of daily, based on a study with good results in Zagreb ("CCTDCP study", n=15 ).
🔹AccuCheck SugarView (Roche) has been successfully launched in Mexico, Brazil, Nigeria, India, Pakistan, Philippines and Vietnam. This "meterless blood glucose meter" received a CE label in 2019 for people with type 2 diabetes and prediabetes who do not use insulin. By downloading the AccuCheck SugarView app, you can use your mobile phone as a blood glucose meter by taking a picture of the AccuCheck Active strip. The app will not give an exact blood glucose value, but a glucose range such as "low" or "very high". For now, there are no plans to bring this technology to Europe.
Voilà, that's it for this month. I hope you learned something new 😀.
Let me know if you have any questions!




