Updates EASD diabetes technology
Sep 25, 2022Every year, the European Association for the Study of Diabetes (EASD) Congress provides the latest updates on diabetes to healthcare providers and researchers around the world. This year was no exception, with several new treatments and advancements being unveiled.
More and more we see the enormous progress in diabetes technology taking an important place at this previously more general diabetes congress.
Here are some of the most exciting developments from this year's EASD Congress, specifically about diabetes technology:
- Omnipod 5 has a CE label
- Mylife Loop with Libre3 is coming to Germany in November 2022
- Combined Glucose and Ketone Monitor from Abbott
- Spectacular results of a full closed-loop system (CamAPS HX) in people with type 2 diabetes
- Control IQ is "the great equalizer"
- The MiniMed 780G does exceptionally well with the elderly + InPen results
- Promising DBL4 pen expected by the end of 2023
- CE label for Tempopen and SoloSmart
- Asian newcomers
- Future ideas
Ready for some hope and inspiration for the future? Then read on quickly!
PS: in the literature there is more and more talk about Automated Insulin Delivery (AID) systems instead of closed-loop systems, which you will also notice in this article.
#1 Omnipod 5 has a CE label
Yes, finally! Omnipod 5 has a CE label! This good news was announced on 9/20/2022, on the 2nd day of the EASD.
Some European countries will be able to start mid 2023, other European countries might have to wait until 2024.
#2 Mylife Loop with Libre3 coming to Germany in November 2022
We already knew they were working on it, but at EASD we also got a date for the launch, which is earlier than expected!
Germany COULD start up on November 1st, 2022, and the Netherlands is next for early 2023.
After that, the mylife Loop with Libre3 will be further rolled out to countries where the Libre3 is available.
(So we hope even more than before that the Libre3 will also be available in Belgium as soon as possible, because that would mean that people in the regular insulin pump convention will finally have a choice between 2 AID systems: the MiniMed 780G or the myLife Loop with Libre3.)
In the meantime, there will also be tests with the first patients who will use this system, as this will be the first AID system with the Libre3.
We know that the Libre sensors sometimes measure lower values than reality, and that there can be differences from sensor to sensor. It is expected that this will have no impact on the functioning of the CamAPS algorithm, but this will be monitored extra closely by Ypsomed. Especially because the Libre3 was recently deemed insufficient by the FDA to be integrated into an AID system.
#3 Combined Glucose and Ketone Monitor from Abbott
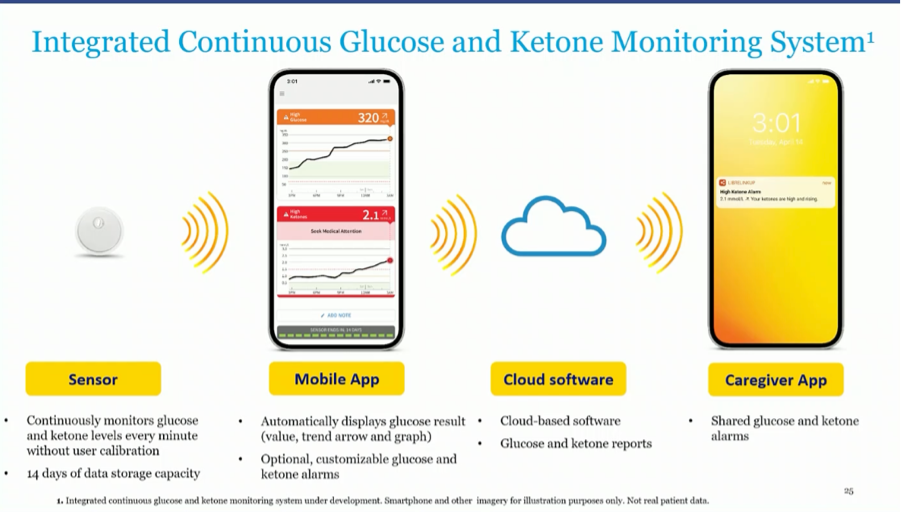
Abbott already announced in June 2022 that they are developing a combined glucose and ketone monitor ("CGM/CKM"), which also received an "FDA Breaktrough Designation".
In this study, they demonstrate that they have the technology to convert their Libresensor into a ketone monitor. Their combined glucose and ketone monitor will be able to be linked to "industry-leading" insulin pumps. It will have the same shape as the Libre3, and connect with an app, cloud software and a tracking app.
It was reiterated at the EASD that the first studies are planned in 2023, after which CE/FDA approval will be requested. So there is a chance that in about 2 years we will have a combined glucose and ketone meter available.
This will become an important sensor as it lowers the risk of ketoacidosis. Many doctors at EASD were already dreaming aloud about the association of an SGLT2 inhibitor with the Automated Insulin Delivery (AID) systems to achieve even better results. This is not recommended now due to the risk of ketoacidosis.
Their Lingo system was no longer communicated. The Lingo franchise was announced with great grace at the beginning of this year. They were sensors in the form of the Libre2, which, in addition to glucose, could also measure ketones, lactate and alcohol.
#4 Spectacular results of a full closed-loop system (CamAPS HX) in people with type 2 diabetes
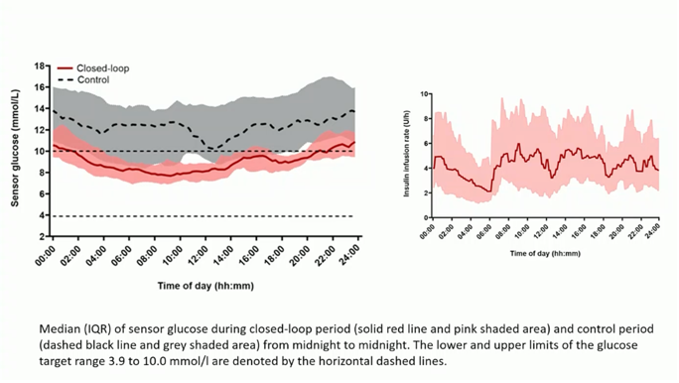
CamAPS HX is an algorithm from CamDiab where you no longer have to enter meals, so it's a "full" AID system.
The algorithm was now studied for the first time in 26 people with type 2 diabetes who were previously taking basal-bolus insulin (mainly without CGM).
- They were randomized into 2 groups: 1 group with the full AID system, and 1 group remained on basal-bolus insulin with blinded CGM.
- After 4 months the TIR of the people in AID increased to 66% instead of 32% in the control group! And this without causing additional hypoglycemia (TBR stable at 0.4%). Their HbA1c dropped from 9.0% to 7.3% (the control group's HbA1c dropped from 9.0% to 8.7%). And all this without these patients having to bolus for their meals!
- Also noteworthy is that the AID system was very well tolerated. The time in auto mode was 92% and each participant said afterwards that he/she would recommend this AID system to others.
- Perhaps rather strange was that these people had a lower glycemia during the day and slightly higher at night. (In hybrid closed-loop systems, we usually see the opposite.)
What does this mean?
Complete AID systems seem to work very well in adults with type 2 diabetes. They are safe and (very) effective. The question will be whether this expensive technology will ever pay for the large population of people with type 2 diabetes on complex insulin regimens, and whether there might be other ways to improve glycemic control by then.
At the EASD we saw not only enormous progress in diabetes technology, but - for people with type 2 diabetes at least - also a lot of new medication, such as GLP1 and combined GLP1/GIP analogues. The latter not only have a spectacular influence on glycemic control, but also on weight.
#5 Control IQ is "the great equalizer"
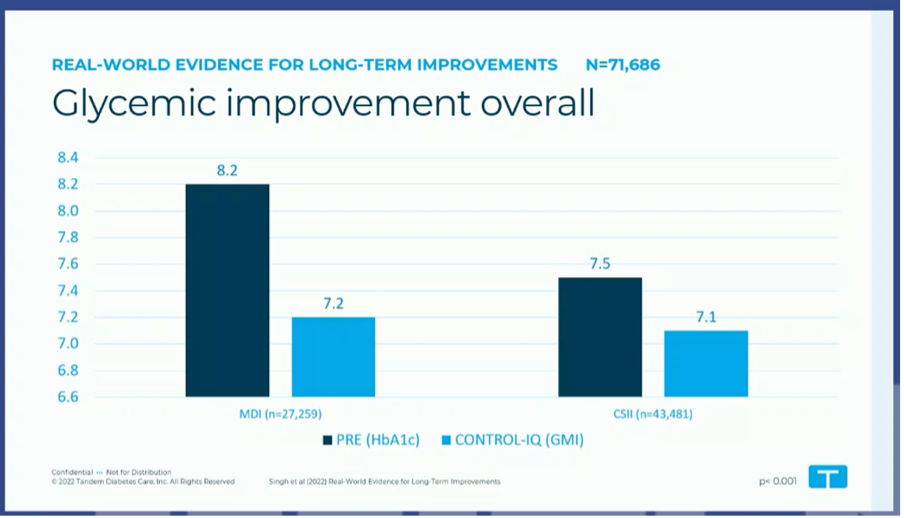
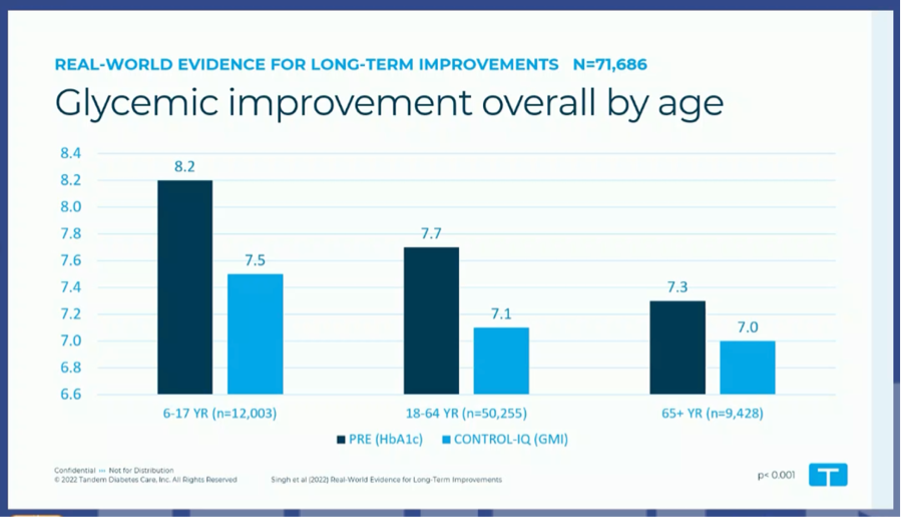
Currently >250,000 people are using Tandem Control IQ, and Tandem's real world datasets are getting bigger and bigger.
At the EASD, results were read from real world data of >71 000 people with type 1 and type 2 diabetes, who used Control IQ for 1 year.
- Control IQ was called "the great equilizer" because the same good results were achieved (HbA1c 7.1–7.2%), regardless of whether you were previously on insulin pens or an insulin pump.
- Also striking were the good glycemic results across all age categories, even in children (HbA1c from 8.2% to 7.5%) and people >65 years (HbA1c from 7.3% to 7.0%).
- (Unfortunately, only the effect on HbA1c was discussed here, not on TIR or TBR, which ould have been interesting.)
#6 MiniMed 780G and InPen: "older" people do even better
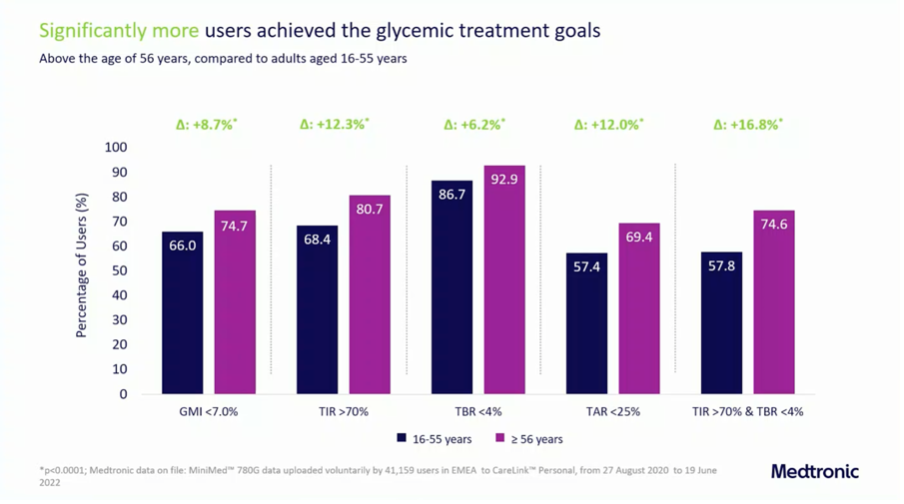
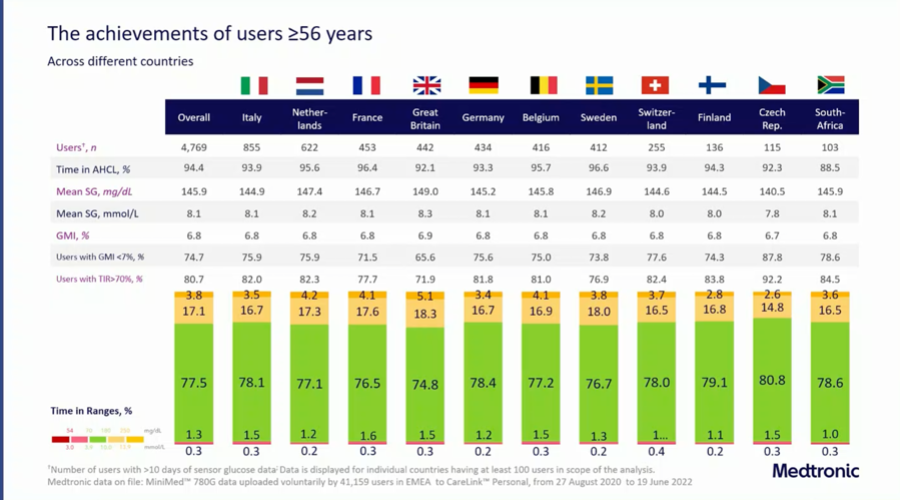
Medtronic presented real world data from 41,159 users of the MiniMed 780G at the EASD.
It was noticeable that the people older than 55 years of age outperform their younger fellows in every way:
- More people achieved a GMI <7%, TIR >70% and TBR <4%,
- and the average TIR among the over-55s was a whopping 77.5%.
- The high time in automode (94.4%) indicates that the system is well tolerated.
Other data from the MiniMed 780G that were discussed were:
- Glycemic results of people taking the MiniMed 780G remained stable for 12 months in the same real-world cohort
- The MiniMed 780G works equally well in people on basal-bolus insulin with an HbA1c ≥8% (ADAPT Trial, Lancet September 2022), and regardless of whether they were previously on basal-bolus insulin or an insulin pump (Acta Diabetologia July 2022).
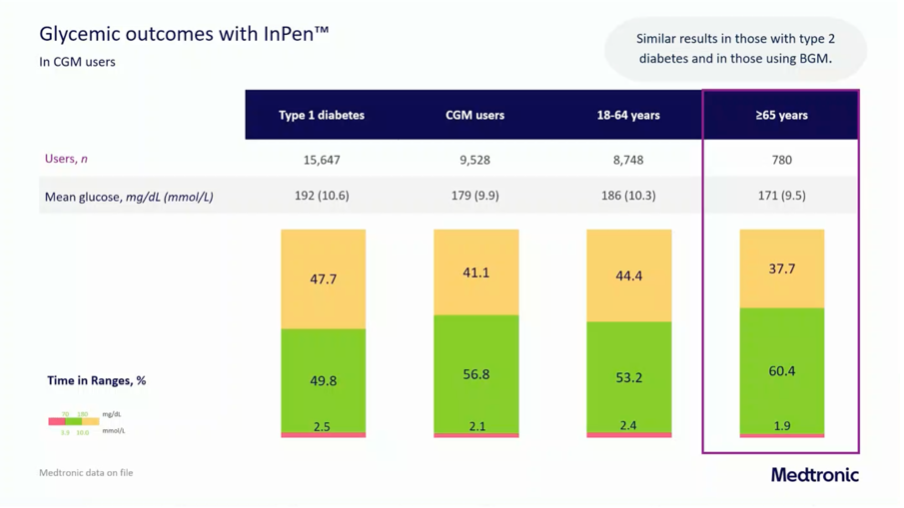
Real world data from the InPen was also shown on >15 000 people who use the InPen.
Again, the "older" people (from 65 years) fared better, with an average TIR of 60.4%.
It is of course noticeable that the results with a smart insulin pen are often much less good than what you can achieve with an AID system.
#7 Promising DBL4 pen expected by the end of 2023

Diabeloop, on the other hand, is very confident that its DBL4pen algorithm will have a very good effect on HbA1c and TIR.
- They tested their algorithm in a computer simulator and found an improvement in TIR from 51.6% to 64.2%. This is a TIR increase of no less than 12%!
- They will soon start a randomized prospective study in 170 people with type 1 diabetes using a combination of the Mallya pen, the Dexcom G6 and their DBL4pen algorithm.
- And they hope to have a CE label by the end of 2023.

To be clear, the DBL4pen is more than a regular smart insulin pen, tracking insulin dose and integrating a bolus calculator.
It contains an algorithm that proposes the correct insulin dose, for both bolus and basal insulin, based on predicted glycemia and past trends.
There has been talk for some time that we could also build a closed-loop system with smart insulin pens in the future, and the DBL4pen is the first example of this!
#8 CE label for Tempopen and SoloSmart

On the first day of the EASD, Biocorp announced that the SoloSmart has a CE label.
- This smart insulin cap is the successor of the Mallya pen cap that consists of 2 pieces.
- The newer SoloSmart only consists of 1 piece and can only be used for the Solo pens from Sanofi.
- The Solosmart would be on the market from early 2023.
It was also reported on the EASD that the Tempo Smart Button has a CE label since last month and would also have an FDA label.
- The Tempo Smart Button will be compatible with the Tempopen for Humalog, Lyumjev and Abasaglar 100 U/ml.
- Lilly has a partnership for this smart insulin cap with companies such as Dexcom, Roche, Glooko and MyDiabby.
- There was no announcement when the Tempo Smart Button would be released.
So it looks like smart insulin pens are making their way to the market, and it's not too soon...
I look forward to finally getting an accurate picture of how much insulin is just being injected. In this way education can be done in a much more targeted way, and the insulin can be dosed in a much safer way!
#9 Asian Newcomers

At the fair I saw some new products (which for the sake of clarity will not be available immediately):
- The iFree CGM from Taiwanese Bionime. The sensor is not described on their website, but it was on display, and 1 of the representatives had it on his arm. The iFree sensor has a lifespan of 14 days and its transmitter is guaranteed for 2 years. It should be calibrated once with a fingerstick at startup and the MARD should be <10%. The start-up is planned for the end of 2022 in Taiwan, and a CE label will be applied for. The app is said to be compatible with Android and iOS. However, they were unable to show a working prototype at the booth, and no publications are planned to further objectify the sensor's accuracy.
- The successor to the Touchcare Nano CGM was shown on the Medtrum stand, under the name of the S10 CGM. It has the same shape as the Libre3, but nothing is known about a possible launch yet. At the moment the Touchcare Nano pump and CGM is available in Denmark, Sweden, UK and the Netherlands (for now only pump). Further roll-outs are planned in the Netherlands (CGM), France and Germany.
- In addition to the Touchcare Nano 200 pump (which can hold 200 E insulin), a Touchcare Nano 300 pump (which can hold 300 E insulin) will also be launched on the market "beginning of 2023".

More exciting is of course the arrival of the hybrid AID system from Medtrum "the Touchcare Nano system".
- At the moment there are 3 people who are already walking around with this, namely a representative from Denmark (here in the photo), one from the UK and one from Sweden.
- A study will soon also start in the UK.
- The Touchcare Nano system would be a PID algorithm (based on the glycemia of the past hours) with a target value of 5.7, 6.1 or 6.7 mmol/l (100, 110 or 120 mg/dl).
- It would give autocorrect boluses and there is also a sport mode.
- There are 2 options to start the system: either enter your total daily dose of insulin and your weight, or (which is recommended) walk around for 2 weeks in the stop-by-low mode and then switch on the auto mode ("Easyloop").
At least at first glance it seems to work. Looking forward to the results of the study!
#10 Future ideas

The EASD often discussed what the future might look like. These were some interesting ideas:
- Prof Thomas Danne (Germany) emphasized that many people prefer to be "naked", they don't want anything on their bodies. So he hoped that we could get as far with smart insulin pens as with the current AID systems. For example, if we could preserve part of the C-peptide, people with type 1 diabetes would need less insulin. Perhaps then basal insulin will no longer be needed, and only bolus insulin can be given with a smart insulin pen. He therefore sees an important role of immunotherapy to protect as much C-peptide as possible.
- Dr Steven Russel (USA) believed in glucose-sensitive insulin, which only works if your blood sugar is above a certain level (eg 50 or 75 mg/dl, or 3 or 4 mmol/l). Beta Bionics hopes to one day combine this type of insulin with glucagon in their iLet Duo. He concluded with "everyone with type 1 diabetes meeting goals for therapy is a realistic target".
Always nice to philosophize about what the future could look like, and what the next breakthrough will be :-)
At the EASD there was a lot of enthusiasm about the possibilities to help people with diabetes even better with the new diabetes technology. These are the 10 highlights about diabetes technology I found most interesting for people with diabetes and their caregivers.
If you want to learn more about where we stand with AID systems, be sure to read the overview article on Medium, which describes in detail the advantages and disadvantages of the 10 most important AID systems. => Click here for the article.
Kind regards,




